PROPERTIES OF MATTER
Unit
Overview
In
the last unit you learned about classifying matter based on its composition. In
this unit you will explore the classification of matter based on its
properties. At the conclusion of the unit, you will be able to differentiate
between chemical and physical properties. You will also be able to classify
matter based on its characteristic properties.
Classifying Matter
Classification of matter
can be done based on properties, with different substances possessing different
properties. Physical properties are those properties which can be observed
without altering the material. Examples of physical properties include color,
solubility, odor, density, conductivity, melting point, boiling point,
malleability, and ductility. Chemical properties are those which
become evident during or after a chemical reaction. Examples of chemical
properties are flammability and reactivity.
Physical Properties of Matter
Some physical properties
of matter, such as color and odor, are easily observable just
from using your senses. When observing the odor of a substance, hold it under
your nose and waft. Wafting is done by repeatedly moving your hand between the
substance and your nose, in the direction of your nose. This is safer than
smelling it directly in case the fumes are especially putrid or noxious.

The solubility of a substance
is determined by how much of a solute will dissolve in a solvent. Sugar is a
substance that dissolves in water; therefore, we can say that sugar is soluble in water.

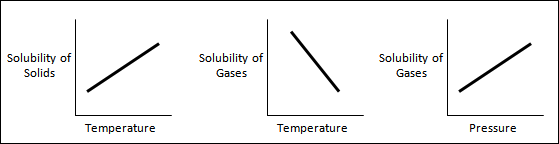
There are two factors
which affect solubility. One factor is the temperature of the solution. Temperature
affects solutes that are gaseous or solid. For many (but not all) solid
solutes, as the temperature increases, solubility also increases. For many
gaseous solutes, as the temperature increases, solubility decreases. The second
factor is pressure. Pressure only affects solutes that are gaseous. As pressure
increases, so does solubility.
Water is known as the universal solvent, because many
substances will dissolve in water.
If a substance does not
dissolve in solution, it is said to be insoluble. For example, if you try
to dissolve sand in water, it will not dissolve. This is because sand is
insoluble in water.
Visit the following
website to learn more about solubility:
http://www.ck12.org/book/CK-12-Chemistry-Second-Edition/r6/section/17.4/
Complete the following
solubility activity to explore the solubility of various substances in water.
Submit as #10 in the assessment portion of the unit.
SOLUBILITY ACTIVITY STUDENT COPY
The density of a substance is
a measure of the compactness of its particles, which is quantified by its mass
per unit volume. The formula for density is as follows:
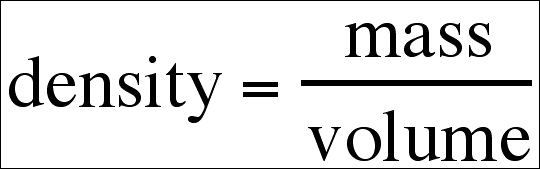
Each substance has its
own unique density, which makes density a characteristic property of matter. A characteristic
property is a property that allows you to identify a substance.
The standard unit for
density is g/cm3. The scale for density is based off the density of
water, which is 1.0 g/cm3. Sometimes you will also see density
expressed in g/mL. One milliliter (mL) is equal to one cubic centimeter (cm3),
so no conversion is required from one unit to the other.
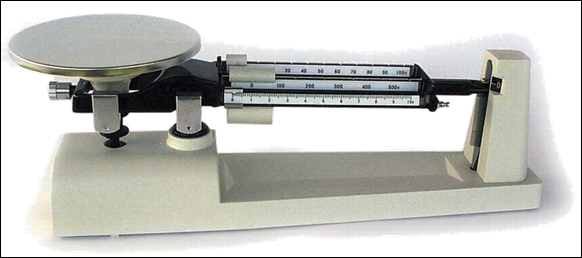
When calculating the
density, you need to know the mass and volume of the substance. A triple beam
balance is conventionally used to measure mass.
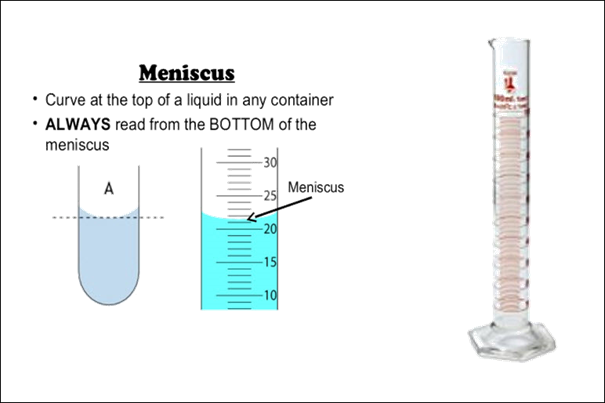
The volume can be
measured in a variety of ways. If the substance is liquid, it can be measured
in a graduated cylinder. Read the volume of the liquid with your eye at the
level of the liquid in the graduated cylinder. The bottom center of the meniscus
curve is the volume of liquid.
If the substance is solid
and a regular shape, such as a cube, you can use a ruler to measure the length,
width, and height of the object and multiply L x W x H.
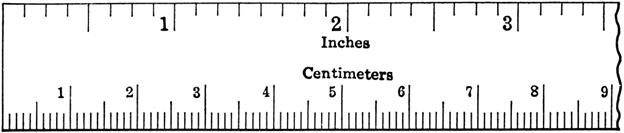
If the substance is solid
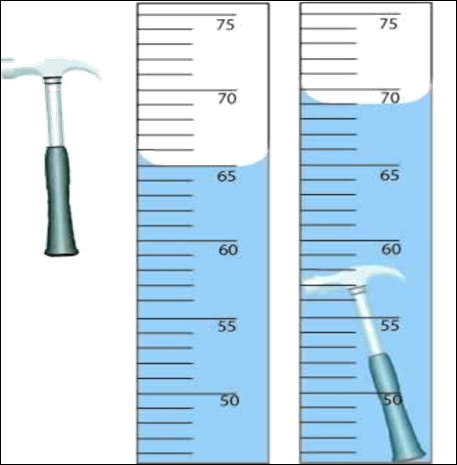
and the shape is irregular or difficult to measure accurately with a ruler, you
can measure the displacement of water in a graduated cylinder. To do this,
place a known amount of water in a graduated cylinder. Measure and record the
amount of water. Then place the object in the graduated cylinder. Measure the
new level of the water. Subtract (final volume – initial volume) to find out
how much water was displaced. This gives you the volume of the object.
Using the formula for
density, solve the following problems. Show your work. Submit your answers for
questions #11-12 in the assessment portion at the end of the unit.
11. You have a coin with
a volume of 10cm3 and a mass of 23g. What is the density of the
coin?
Conductivity is a measure of a substance’s ability to conduct heat
or electricity. Because certain types of matter will conduct electricity,
conductivity is another characteristic property of matter. As a rule, metals
are good conductors and nonmetals are poor conductors. Recall from the
electricity unit that some substances are semiconductors, and are able to
conduct electricity under certain conditions.
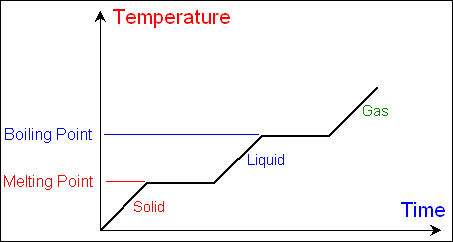
The melting point of a
substance is the temperature at which a solid substance changes to a liquid.
The boiling
point of a substance is the temperature at which a liquid substance
changes to a gas. The melting point and boiling point of various substances are
unique to the substances, which makes these characteristic properties of
matter. You will learn more about states of matter, phase changes, and melting
and boiling points in the next unit.
The malleability of a
substance refers to its ability to be flattened and/or rolled into thin sheets.
A good example of a malleable material is aluminum. You are familiar with
aluminum foil, which is pressed into thin sheets and rolled.

Ductility is the ability of a substance to be drawn into a
wire. Copper is an example of a ductile substance.

Generally, metals are
both malleable and ductile. Nonmetals are more brittle and not as malleable or
ductile as metals.

Malleability Quick Lab:
If you have some aluminum foil, take a sheet of it and roll it into a ball.
Then, very carefully, try to unroll it and flatten it back into its original
shape. It will probably appear wrinkly, but if you take your time, you should
be able to flatten it again.
Video
Clip: Physical Properties
Watch the following video
clip on physical properties of matter and complete the guided notes. Complete
the document and submit as #13 in the assessment portion at the end of the
unit.
Chemical Properties of Matter
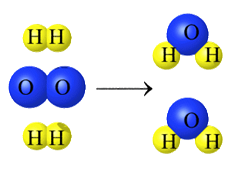
To reiterate, a reaction
must occur in order to observe chemical properties of matter. When two or more
substances combine and form new substances, a chemical reaction has
occurred.
One chemical property of
matter is flammability. This is a substance’s ability to catch fire. When
something burns, it undergoes a chemical change.

Reactivity is another
chemical property of matter. Reactivity is the tendency of a
substance to undergo a chemical change by reacting by itself or with other
substances to form new substances.

Quizlet Vocabulary