CLASSIFICATION OF MATTER
Unit Overview
In
this unit, you will learn some of the ways in which matter is classified. You will learn how to define matter and
classify it as an element, compound, or mixture. You will be able to
differentiate between homogeneous and heterogeneous substances. You will
investigate different types of mixtures.
What
is Matter?
All substances around us
are composed of matter. Matter is defined as anything that
has mass and takes up space. Water is an example of matter. It has mass and
occupies space. Helium gas is another example of matter. Although helium may
not seem to have mass or be taking up space, you can prove it takes up space by
filling up a balloon with helium. You could measure the mass of the balloon
before and after filling it to get the mass of the helium.
Check out the following
video to learn more about matter, and how it is different from energy:
Classification of Matter
Matter is classified into
categories based on how it is composed. Three categories for classifying matter
according to composition include elements, compounds and mixtures. Mixtures can
be further categorized into solutions, suspensions, and colloids. In order to
understand the similarities and differences among elements, compounds, and
mixtures, you must first understand the most basic building blocks of matter:
atoms.
An atom is the smallest
building block of matter that cannot be broken down further and still retain
the properties of that substance. A molecule is a group of atoms held
together by a chemical bond. There can be one or more types of atoms in a
molecule. If a substance has only one type of atom, or only one type of
molecule, it is known as a pure substance. You will become much
more involved in your comprehension of atoms and molecules in the following
units, but a basic understanding helps when learning about elements and
compounds.
Elements and Compounds
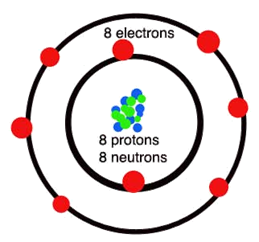
An element is a pure
substance that contains only one type of atom throughout the material. Examples
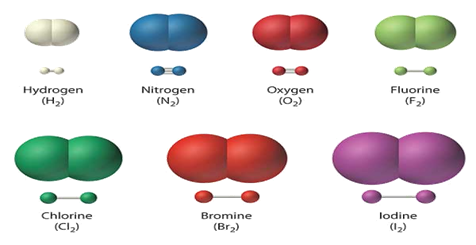
of elements include oxygen, carbon, and mercury. Some elements exist as single
atoms, where others exist as diatomic molecules. This means that instead of
being composed of single atoms, the element has two of the same atom fused
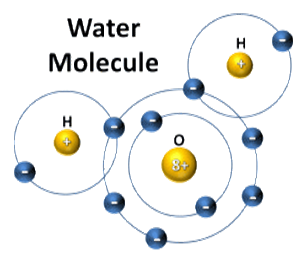
together in a molecule. A compound is a pure substance that is
made when two or more elements are chemically bonded together. Examples of
compounds include water and sodium chloride.
|
Oxygen atom: |
|
|
|
Diatomic molecules: |
|
|
|
Water molecule: |
|
|
Heterogeneous vs
Homogeneous
Matter can be further
classified based on whether the substance’s particles are homogeneous or
heterogeneous. Homogeneous substances have the same particles throughout. Heterogeneous
substances have different particles in a sample. Elements and compounds are
both types of homogeneous substances.
Mixtures
Mixtures are made when two or more elements or compounds are
physically combined. Mixtures are not pure substances because they are not the
same composition throughout. Mixtures are different from compounds because the
components of a mixture are physically combined, but the components of a
compound are chemically bonded. Similarly, mixtures can be physically
separated, but compounds cannot.
There are different types
of mixtures, which are categorized based on the arrangement and size of
particles in the mixture. First, mixtures can be either homogeneous or
heterogeneous. A heterogeneous mixture has particles that are not
evenly dispersed. Examples of heterogeneous mixtures include salad and trail
mix. A homogeneous mixture has particles that are evenly dispersed
throughout the sample. Examples of homogeneous mixtures are lemonade and
mayonnaise.
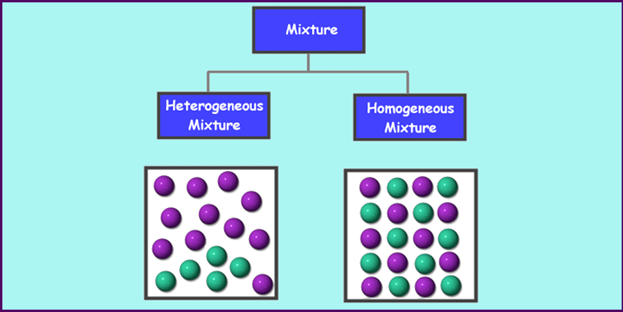
Homogeneous mixtures are
further divided into types known as solutions, suspensions, and colloids.
Watch the following video
on solutions, suspensions, and colloids, and complete the guided notes as you
watch. You will attach your answers in question 11.
|
|
Solution |
Suspension |
Colloid |
|
Particle size |
|
|
|
|
Particle
behavior (what happens to the mixture over time?) |
|
|
|
|
Examples |
|
|
|
A solution is one type of
homogeneous mixture that is sometimes mislabeled as a compound. A solution
is made when a solute (the substance being dissolved) is mixed into and
dissolved into a solvent (the substance doing the dissolving). An example of a
solution is salt water. The salt is the solute and the water is the solvent.
The solution is the combination of solute and solvent, and in this example the
solution is the salt water that is made. The salt dissolves and the particles
evenly disperse throughout the water in the solution. Because the salt water is
a physical combination of substances, and the salt and water are not chemically
bonded, it is a mixture and not a compound.
|
Solute (salt) |
Solvent (water) |
Solution (salt water) |
|
|
|
|
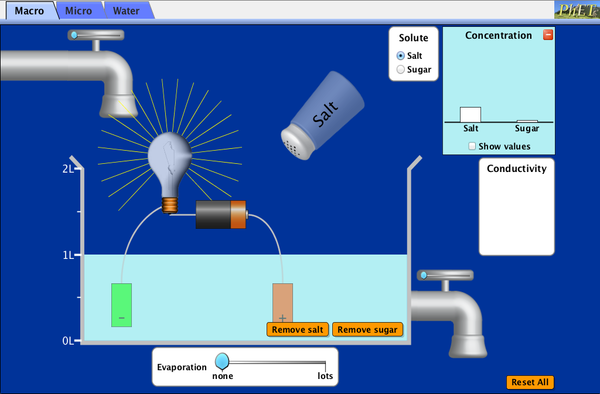
PhET Simulation
What happens when sugar
and salt are added to water? Explore the PhET simulation to further explore
solutions. Pour in sugar, shake in salt, and evaporate water to see the effects
on concentration and conductivity. Zoom in to see how different sugar and salt
compounds dissolve. Zoom in again to explore the role of water.
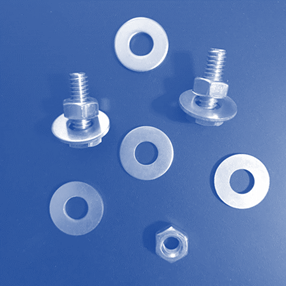
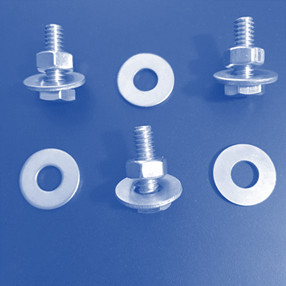
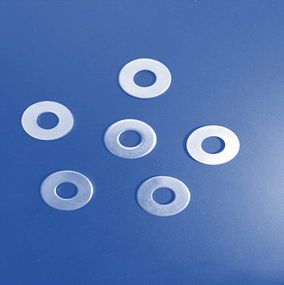
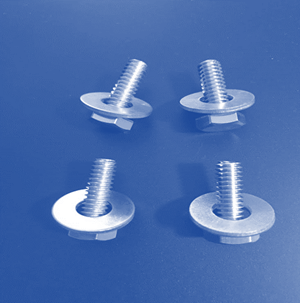
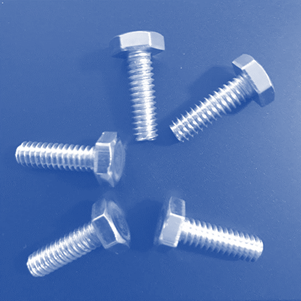
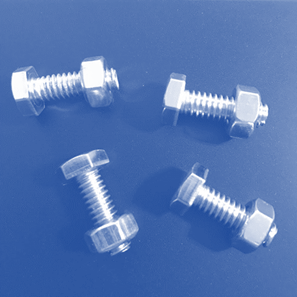
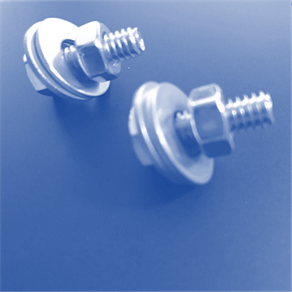
Laboratory
Assessment: Nuts and Bolts
With the information you
have learned in the unit, apply what you know to complete the Nuts and Bolts
lab test. Download the attached document and use the images below to complete
the test. Here are the names of the different pieces used in the samples, so
you may correctly refer to them:
|
Nut: |
Bolt:
|
Washer: |
|
|
|
|
Part I
SUDENT COPY_NUTS AND
BOLTS ASSESSMENT
Answer the questions in
the first part of the assessment based on what you’ve learned in the unit.
Part II
Now, evaluate these
samples. Decide whether each sample contains atoms of an element, molecules of
an element, molecules of a compound, a heterogeneous mixture, or a homogeneous
mixture.
|
Sample 1:
|
Sample 2:
|
|
Sample 3:
|
Sample 4:
|
|
Sample 5:
|
Sample 6:
|
|
Sample 7:
|
Sample 8:
|
|
Sample 9:
|
Sample 10:
|
Part III
Finally, complete the
reflection questions in the third part of the assessment. Attach your Nuts and
Bolts assessment in question 13.
Unit Vocabulary
Review the vocabulary
before completing the unit assessment.