RADIANT ENERGY and ELECTROMAGNETIC
RADIATION
Unit Overview
In this unit you will
learn about radiant energy and electromagnetic radiation, and how these topics apply
to our everyday lives. It is important to note that some concepts and questions
in this unit will come from the NASA website investigation.
Radiant Energy

Radiant energy is a form of energy that travels through
electromagnetic waves. It does not require a medium to travel, and it spreads
in all directions. Examples of radiant energy are sunlight and lightbulbs. In
order to comprehend radiant energy, you need to have an understanding of
electromagnetic waves and electromagnetic radiation.
Electromagnetic Waves
There are various types
of waves around you: some are able to be detected with the eye while others are
invisible. Examples of types of waves in our environment include: radio, radar,
X -ray, infrared, microwaves, ultraviolet, and gamma rays. Collectively, they
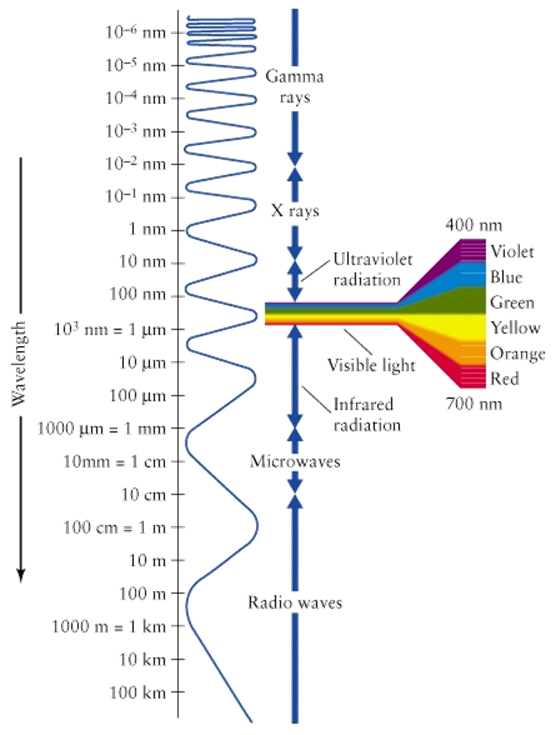
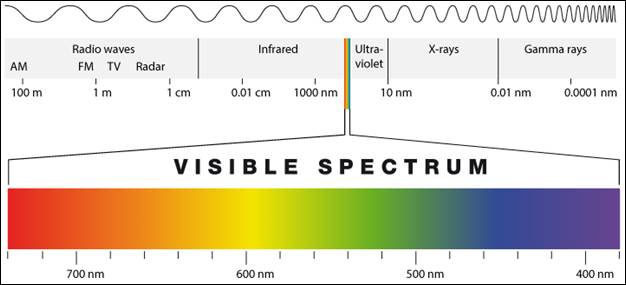
are known as electromagnetic waves and make up the electromagnetic spectrum.
Each type of wave has different properties, such as its wavelength and amount
of energy emitted. The types of electromagnetic waves are organized along the
electromagnetic spectrum, seen below:
Video Clip:
Properties of Electromagnetic Waves
Learn the basics of electromagnetic
waves by watching the following video clip and completing guided notes:
Watch the following video clip and complete the guided notes.
![]() Properties
of Electromagnetic Waves
Properties
of Electromagnetic Waves
The Electromagnetic Spectrum
The electromagnetic spectrum
classifies waves based on their wavelength and frequency. In order to
understand these terms, it is necessary to become familiar with basic wave
terminology.
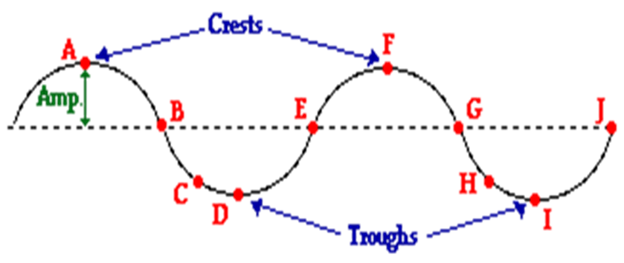
The top of a wave is
called the crest, noted as A and F in the illustration below. The bottom
of a wave is the trough noted as D and I. The distance between two crests (A and
F) is called the wavelength and, the frequency is the number of waves
that pass a given point in one second.
Imagine throwing a stone
into a pond of water, and the energy created by that stone as it enters the
water generating waves. If you count the number of waves that pass an
established point in a measured amount of time, you can determine the
frequency.
If ten waves passed the
established point in five seconds, the frequency is found by dividing the
number of waves by the time.

Frequency =
Waves/Time = 10/5 = 2
Frequency is measured in
Hertz (Hz), so the frequency of the waves generated in the water is 2Hz.
The electromagnetic
spectrum classifies waves from shortest wavelength to longest wavelength, which
is measured in meters (or as small as nanometers for the shortest wavelengths).
Electromagnetic waves are
different from other waves (liquid and sound) because they do not require a
medium (matter) for transmission, but are capable of traveling through empty
space (vacuum). A wave will change speeds when it travels through
different media. A person can run fastest through an air environment, slower
through water (liquid), and not at all through a solid. The same is true for
waves. The sun’s rays travel fastest in space because there are no particles of
matter to slow the wave down. Once the light reaches Earth’s atmosphere, it
must travel through air which slows down the light speed. The light may then
hit water (liquid) and be slowed to a lower speed. These changes in media can
also be a way to guide light.
NASA Website
Investigation: Electromagnetic Spectrum
Goto the website above
to complete the NASA website investigation on the electromagnetic spectrum. As
you read through each page, write a one- or two-sentence summary of each
section. Click “next” at the bottom of each page to advance to the next topic until
you get through the last page.