![j0198419[1]](SCIPSU11Thermal_Energy_image003.png)
THERMAL ENERGY, POTENTIAL ENERGY
AND KINETIC ENERGY
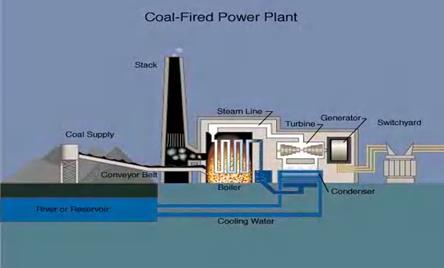
A definition of energy is the capability to do work. There are different forms of energy that matter can possess. One form of energy is thermal energy which can be thought of as heat energy. When a substance is hot it is often described as having thermal energy is actually the molecules moving faster and the electrons moving to higher levels. This motion is called kinetic energy. Thermal energy at the atomic level causes atoms in a compound to vibrate. When water is boiled, you can see the water move. The more heat that is added to a substance, the more the particles of that substance move. This molecular motion produces heat, heat is molecular motion. When cold water is put on the stove to boil the burning gas is adding kinetic energy to the water, the molecules move progressively faster and faster, the electrons move to higher levels until the water (liquid) expands into steam (gas). This fast motion of the atoms is kinetic energy. Steam has a lot of kinetic energy. Steam is often used to do work, for instance trains, electric power plants, and boats. The rate at which thermal radiation is absorbed or emitted by a system depends on its temperature, color, texture and exposed surface area.
|
|
|
|
|
|
||
The opposite is true for cooling a substance. The colder a substance becomes, the less the particles move because they have lost energy. Ice has less kinetic energy than liquid water. We add ice to our drinks to remove kinetic energy. We have air conditioners to remove kinetic energy from our house on hot days.
Do not
confuse heat with temperature. Two
masses of matter can have the same temperature but possess different amounts of
heat. It takes more heat to warm a
gallon of water than it does to warm a cup of water. If both the cup and pot are warmed to the same
temperature, the gallon possesses more energy.
The transfer
of energy from one object or system to another involves work. This clip gives the formula for work
and examples.
![]() Elements of Physics: Energy: Work and Power
Elements of Physics: Energy: Work and Power
Kinetic Energy
Measuring temperature, with a thermometer, is a way to measure the amount of kinetic energy possessed by an atom. Kinetic energy is the energy level of a moving object. As heat is added to a substance, the particles gain energy, move faster, and, therefore, have more kinetic energy. A substance with a higher kinetic energy level will also have a higher temperature. The only time that heat can be added without changing temperature is when a phase change occurs. Phase changes are processes such as evaporating and melting. A pot of water placed on a stove will increase in temperature until the water begins to boil. At the boiling point, a phase change is occurring as the water is turning from a liquid to a gas, evaporation. The temperature of the water will not change since all of the heat energy goes into changing the phase and none is left to warm the water. The opposite phenomenon happens during freezing, energy is removed changing liquid water to solid ice and no energy is left to make the water colder until all the water is frozen then, the ice can get colder.
Earlier, it was stated that substances and particles can
have kinetic energy. The formula for
kinetic energy is K.E. = ½ mv2.
(m = mass and v = velocity) By
looking at the equation, you can see there are two ways to increase
kinetic-energy, by changing the mass or changing the velocity. For example, a cup of water has a certain KE,
to increase the KE you would need to add more water (mass) or increase the
speed of the molecules by adding heat.
Applying the formula in an everyday example-a small car traveling very
fast will have the same kinetic energy as a large truck traveling  slowly. The car has a high velocity and a small mass,
while the truck has low velocity and a large mass causing both moving objects
to have the same kinetic energy.
slowly. The car has a high velocity and a small mass,
while the truck has low velocity and a large mass causing both moving objects
to have the same kinetic energy.
To learn more about kinetic energy, click on this link:
Potential Energy
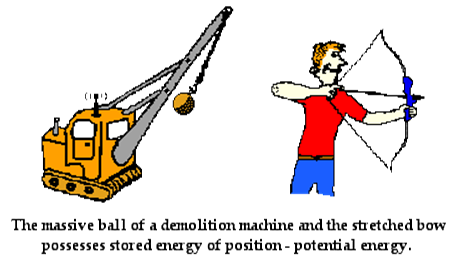
The second form of energy an object or substance can possess
is potential energy. Potential energy is often thought of as energy of position
or rest energy. Potential energy is in
place because an object has potential to move.
It is often called Gravitational Potential Energy because of its height
above the surface of the Earth. The
higher an object is, the more energy it can gain as it
falls towards the Earth. A book sitting
on a table possesses potential energy since it has the potential to fall off of
the table. Why might the book fall? There is a force pulling on the book known as
gravity. The formula for potential
energy is P.E. = mgh (m = mass, g = gravity and h =
height) since gravity on earth is a constant, there are two things that can
increase potential energy: mass and
height. The height is measured from a
reference point that is considered to be at a point of zero energy. This point can change with the
situation. It can be the ground under
your feet, the floor of the class room, even the top of your desk. A student on the 3rd floor of a
building has more potential energy compared to another student on the 2nd
floor. A 200 gram weight sitting on a
table has more potential energy than a 100gram weight on the same table.
To learn more about potential energy, click on this link: BrainPOP Potential Energy
Learn about conservation of energy with a skater dude! Build tracks, ramps and jumps for the skater and view the kinetic energy, potential energy and friction as he moves. You can also take the skater to different planets or even space!