Unit 16:
Covalent Nomenclature
Unit Overview:
In
the last unit, you examined the chemical nomenclature of ionic compounds,
learning how to write ionic formulas and name them. In the laboratory, you conducted a laboratory
to identify the components of unknown ionic compounds.
In
this unit, you will engage in the same learning process, examining the chemical
nomenclature of covalent molecules.
How is a covalent molecule different
from an ionic compound?
An ionic compound is a substance that is
held together by an ionic bond,
which refers to the transfer of electrons between atoms. It is formed between
positive and negative ions, which are formed by metals and nonmetals. The chemical formula is a ratio formula that
shows the ratio of ions that result in a neutral compound.
An covalent molecule is a substance that
is held together by a covalent bond,
which refers to the sharing of electrons between atoms. Because electrons are
shared, not transferred, no ions are formed; instead, a singular structure in
which elements overlap electron clouds is formed. Molecules form between two or
more nonmetals. The chemical formula is an actual formula that shows how many
atoms share electrons within that structure.
What are the Greek Prefixes?
Covalent
nomenclature utilizes Greek prefixes to indicate the numbers of elements
involved in a molecule.
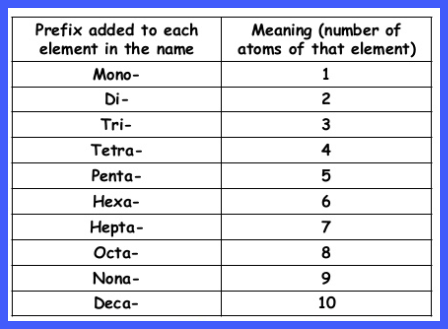
The number of atoms that are involved
in the molecule is indicated by Greek Prefixes:
|
Number of Atoms |
Greek Prefix |
|
1 |
mono- |
|
2 |
di- |
|
3 |
tri- |
|
4 |
tetra- |
|
5 |
penta- |
|
6 |
hexa- |
|
7 |
hepta- |
|
8 |
octa- |
|
9 |
nona- |
|
10 |
deca- |
Some
Special NOTES:
1.) The prefix
mono- is omitted from the description of the first element in the molecule.
2.) The final
vowel in a prefix is often dropped before a vowel in a stem name.
Example: CO is carbon monoxide, NOT monocarbon monooxide!
How is a covalent formula written?
In covalent nomenclature, by convention, the less
electronegative atom is placed first, followed by the more electronegative
atom. Remember that electronegativity describes the pull of electrons within a bond. So
let’s review the periodic trends of electronegativity that you explored in unit
11. . .
Within a
family, the electronegativity decreases as you move down the family.
Within a
period, the electronegativity increases as you move across a period.
|
To write formulas, given the name: |
|
Let’s Try Some! Write the formula for each of
the following covalent molecules:
1.
nitrogen dioxide
|
Step 1: Convert the names of the elements to symbols. nitrogen is the symbol N. 0xide is the element oxygen,
with the symbol O. |
|
Step 2: Convert the prefixes to subscripts. No prefix in front of
nitrogen means that there is 1 nitrogen atom in the molecule. The prefix di- in front of oxide means that there are 2 oxygen atoms in the molecule. |
|
Step 3: Write the formula. NO2 |
2.
carbon disulfide
|
Step 1: Convert the names of the elements to symbols. carbon is the symbol C. sulfide is the element
sulfur, with the symbol S. |
|
Step 2: Convert the prefixes to subscripts. No prefix in front of carbon
means that there is 1 carbon atom in the molecule. The prefix di- in front of sulfide means that there are 2 sulfur atoms in the molecule. |
|
Step 3: Write the formula. CS2 |
3.
silicon trichloride
|
Step 1: Convert the names of the elements to symbols. silicon is the symbol Si. chloride is the element
chlorine, with the symbol Cl. |
|
Step 2: Convert the prefixes to subscripts. No prefix in front of
silicon means that there is 1 silicon atom in the molecule. The prefix tri- in front of chloride means that there are 3 chlorine atoms in the molecule. |
|
Step 3: Write the formula. SiCl3 |
4. diphosphorus pentoxide
|
Step 1: Convert the names of the elements to symbols. phosphorus is the symbol P. Oxide is the element oxygen,
with the symbol O. |
|
Step 2: Convert the prefixes to subscripts. The prefix di- in front of
phosphorus means that there are 2 phosphorus atoms in the molecule. The prefix pent(a)- in front of oxide means that there are 5 oxygen atoms in the molecule. |
|
Step 3: Write the formula. P2O5 |
|
To name compounds, given the formula: |
|
Let’s Try Some! Write
the name of each of the following covalent compounds:
1.
N2O4
|
1st Atom: Convert subscript to prefix, a symbol to name. ●
Subscript 2 is prefix di- ●
Symbol N is nitrogen |
|
2nd Atom: Convert subscript to prefix, symbol to a name, with -ide ending. ●
Subscript 4 is prefix tetra- ●
Symbol O is oxygen → oxide. |
|
The name is dinitrogen tetroxide. |
2.
CI2
|
1st Atom: Convert subscript to prefix, a symbol to name. ●
No subscript is an understood 1, which means the prefix mono-, but
this prefix is dropped from the first element. ●
Symbol C is carbon. |
|
2nd Atom: Convert subscript to prefix, a symbol to name, with -ide ending. ●
Subscript 2 is prefix di- ●
The symbol I is iodine →
iodide. |
|
The name is carbon diiodide. |
3.
CCl4
|
1st Atom: Convert subscript to prefix, a symbol to name. ●
No subscript is an understood 1, which means the prefix mono-, but
this prefix is dropped from the first element. ●
Symbol C is carbon. |
|
2nd Atom: Convert subscript to prefix, a symbol to name, with -ide ending. ●
Subscript 4 is prefix tetra- ●
Symbol Cl is chlorine →
chloride. |
|
The name is carbon tetrachloride. |
4.
BF3
|
1st Atom: Convert subscript to prefix, a symbol to name. ●
No subscript is an understood 1, which means the prefix mono-, but
this prefix is dropped from the first element. ●
Symbol B is boron. |
|
2nd Atom: Convert subscript to prefix, a symbol to name, with -ide ending. ●
Subscript 3 is prefix tri- ●
Symbol F is fluorine → fluoride. |
|
The name is boron trifluoride. |
Watch
the following video for a verbal and visual description of writing formulas and
naming of covalent molecules: Covalent
Nomenclature
Practice: Complete this online practice quiz of naming and writing
covalent molecules.
ChemLab: Identifying Nutrients
Overview:
In
this lab, you will not directly explore covalent nomenclature. Instead, you will analyze food samples in
order to determine if the food is a carbohydrate, a protein, or a lipid, all of
which are held together by covalent bonds. You will perform multiple tests to
identify these nutrients.
Directions:
1. Download the Student
Exploration and Vocabulary sheets
for the Identifying Nutrients.
2. Familiarize yourself with the
words on the vocabulary sheet.
3. Log-in to your Explore Learning account.
4. Click on “Identifying
Nutrients” and launch the gizmo.
5. Answer the Prior Knowledge
Question.
6. Practice using the Gizmo,
using the Gizmo warm-up instructions.
7.
After you are comfortable using the Gizmo, begin the
activity. Use the lab sheet as a guide to complete the 2 activities:
a. Activity A: Identifying
Nutrients
b. Activity B: Nutrients and
Food Types