Unit 15: Ionic
Nomenclature
Unit Overview:
In
the previous 2 units, you built on your understanding of both the electronic
structure and the periodic table to explore the role of the electron in
chemical bonding. Ionic bonding is the
result of a transfer of electrons; covalent bonding is the result of a sharing
of electrons.
In
the next 2 units, you will explore the writing of formulas and the naming of
compounds that result from these ionic and covalent bonds.
What is Chemical Nomenclature?
Our
world is made up of millions of substances - substances that occur naturally
and substances that are synthesized by us.
How do we keep track of all of these substances? Chemical
nomenclature refers to the systematic naming of and writing the formulas of chemical substances. It needs to be systematic, so that scientists
can be sure to accurately describe the substances that they are using in
reactions, regardless of language.
Chemical nomenclature, which builds from the universal symbols of
elements on the periodic table, becomes
a universal language for chemists. Different sets of rules are used to govern
the naming and writing formulas for different types of substances. These rules are developed by the International Union of Pure and Applied Chemists and is often referred to as the IUPAC System of Nomenclature. There are rules that govern the writing of
ionic compounds and a different set of rules for the writing of covalent
molecules.
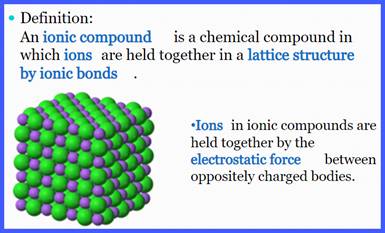
What makes up an ionic compound?
Remember
that an ionic compound is a
substance that is held together by an ionic
bond, which refers to the transfer of electrons between atoms. When the
atom transfers electrons, an ion is
formed. An ion is a charged atom or group of atoms. It is important to
remember that:
1. Ions can either be positively
or negatively charged, depending on whether or not electrons have been gained
or lost:
a. A cation is s positively-charged ion because it has lost electrons.
Metals form cations.
b. An anion is a negatively-charged ion because it has gained electrons.
Nonmetals form anions.
2. Ions can be formed by single
atoms or by groups of atoms.
a. A monatomic ion is formed by a single atom gaining or losing
electrons.
b. A polyatomic ion is formed when a group of atoms has gained or lost
electrons.
How do you know the charge of ions?
The
charge of the ion, also sometimes referred to as the oxidation number, indicates how many electrons have been lost or
gained by the atom or group of atoms in order for it to achieve greater
stability. When electrons have been
lost, the overall charge is positive; when electrons have been gained, the
overall charge is negative.
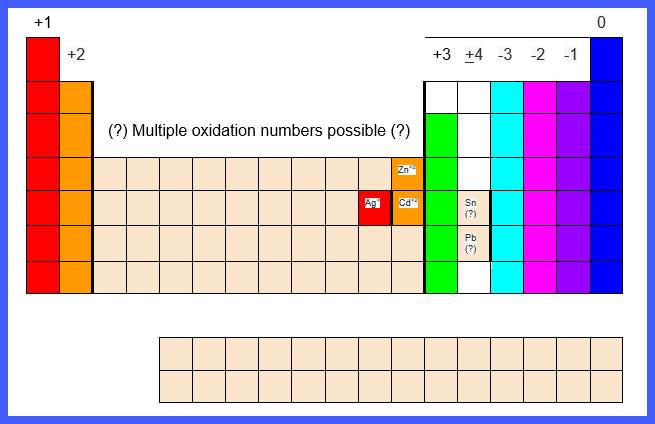
For
monatomic ions, the charge of the ion can generally be determined by its
location on the periodic table:
● Group 1 elements (alkali
metals and Hydrogen) have a +1 charge
● Group 2 elements (alkaline
earth metals) have a +2 charge.
● Group 13 metals have a +3
charge
● Group 15 nonmetals have a -3
charge
● Group 16 elements have a -2
charge
● Group 17 elements (halogens)
have a -1 charge
● Group 18 elements (noble
gases) are stable and do not enter into ionic bonds
● Groups 3 - 12 elements
(transition elements) can have multiple oxidation numbers
○ There are several exceptions:
■ Silver, Ag, has an oxidation
number of +1
■ Cadmium, Cd, has an oxidation
number of +2
■ Zinc, Zn, has an oxidation
number of +2
■ Tin, Sn, and Lead, Pb, both
have multiple oxidation numbers but are not transition elements
The
Periodic Table chart below summarizes the charges of monatomic ions:
The
following chart also summarized the names and charges of common monatomic ions:

There
are two essential things to notice about these monatomic ions:
1. For elements that can have
more than one oxidation number, their charge is included in the name as a Roman
numeral.
2. For anions, the name of the ion
is not the same as the element name; instead, they end in -ide.
For
polyatomic ions, the periodic table cannot be directly used to know the names
and charges. Instead, names and charges
can be looked up.
The
following chart summarizes the names and charges of common polyatomic ions:
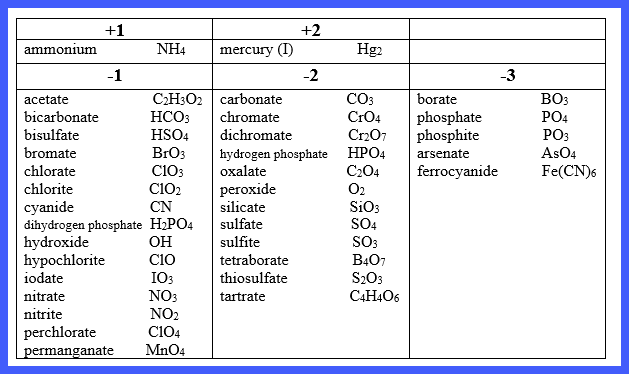
There
are two critical things to notice about these polyatomic ions:
1. The most common polyatomic
ions are anions.
Exception: ammonium, NH4+1;
mercury (I), Hg2+2
2. The names of most common
polyatomic ions end with -ate or or -ite.
Exceptions: cyanide, CN-1; hydroxide, OH-1;
peroxide, O2-2; ferrocyanide, Fe(CN)6-3
How is an ionic formula written?
The
writing of ionic formulas is governed by the principle of electric neutrality, which describes that a compound
is formed so that it has no overall charge.
Therefore, the total amount of positive charge must equal the total
amount of negative charge. So, in order to write formulas correctly, it is essential
to know the charges of the ions that make it up.

Let’s Try Some! Write the formula for each of
the following ionic compounds:
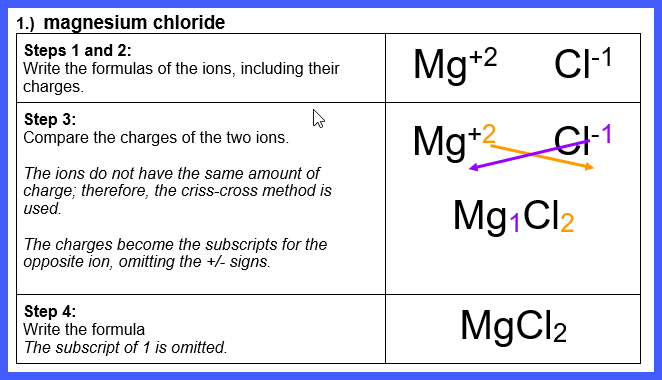
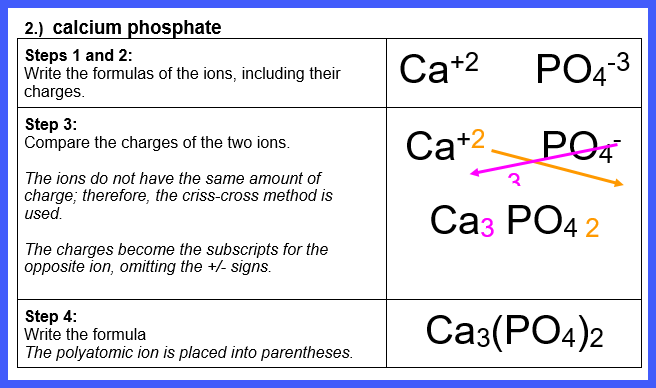
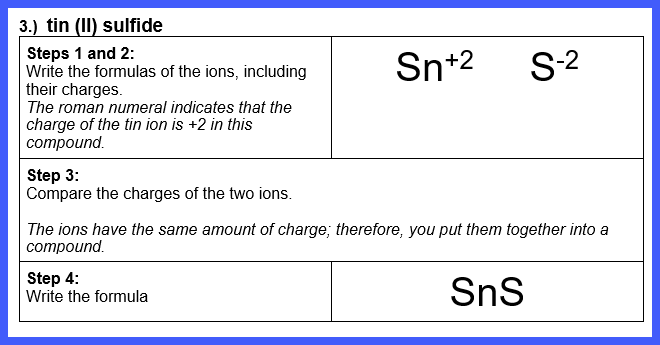
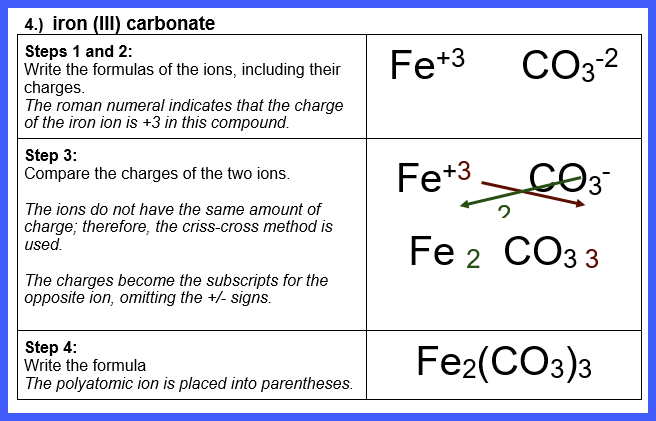
Watch
the following video for a verbal and visual description of writing formulas of
ionic compounds. Writing Ionic Formulas
Practice
1:
Complete this online practice quiz of writing the formulas of ionic
compounds from their names.

Let’s Try Some! Write the name
of each of the following ionic compounds

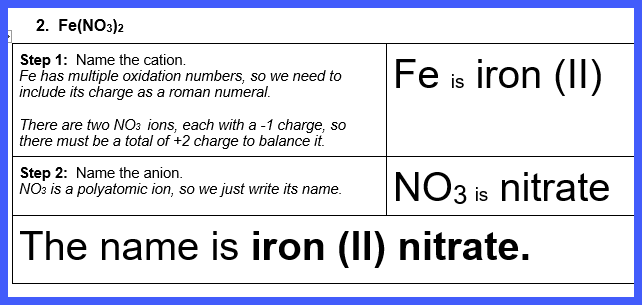


Watch
the following video for a verbal and visual description of naming ionic
compounds from their formulas: Naming Ionic
Compounds
.
Practice
2: Complete this online practice quiz of naming and writing ionic
formulas.
ChemLab: Mystery Powder Analysis
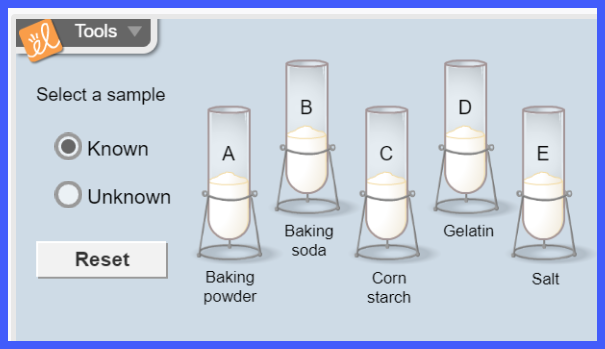
Overview:
In
this lab, you will not directly explore ionic nomenclature. Instead, you will use scientific methods to
identify an unknown substance. You will perform multiple experiments using
several common powders (that include ionic compounds). The results of the
research on the known powders will be used to analyze and identify several
unknowns. The unknowns can be a single powder or a combination of the known
powders.
Directions:
1. Download the Student Exploration and Vocabulary sheets for the Mystery Powder Analysis.
2. Familiarize yourself with the
words on the vocabulary sheet.
3. Log-in to your Explore
Learning account.
4. Click on “Mystery Powder
Analysis” and launch the gizmo.
5. Answer the Prior Knowledge
Question.
6. Practice using the Gizmo,
using the Gizmo warm-up instructions.
7.
After you are comfortable using the Gizmo, begin the
activity. Use the lab sheet as a guide to complete the 2 activities:
a. Activity A: Known Substances
b. Activity B: Unknown
Substances