| |
Click on the link above to get an overview of Acids and Bases.
|
|
|
| |
| |
|
| |
|
| 3) A beaker full of acid is added to a beaker full of base. The pH of the base will: |
|
|
| Correct! |
|
| |
|
| 4) An unknown substance is added to a solution and the pH increases. The substance is a: |
|
|
| Correct! |
|
| |
|
| 5) A solution with a pH of ___ is considered to be neutral. |
|
|
| Correct! |
|
| |
|
| 6) Look at the picture below and list two acids.
|
 |
3980 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
| 7) Look at the picture below and list two bases.
|
 |
3968 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
The pH Scale
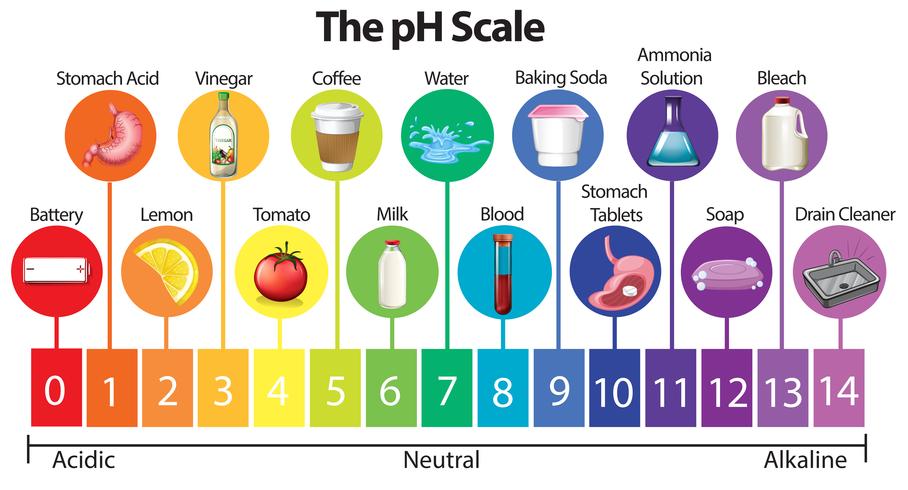
|
|
|
| |
Click on the picture above to get an overview of the pH Scale.
|
|
|
| |
| |
|
| |
|
| |
|
| |
|
| 12) Acid Property: Acids destroy the chemical properties of bases.
Base Property: Bases destroy the chemical properties of acids.
______________ is the name for this type of reaction. |
|
|
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
Acid-Base Chemistry
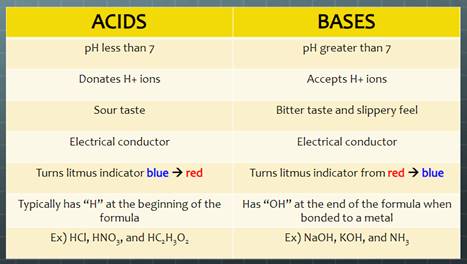
|
|
|
| |
Click on the picture above to get an overview of Acid-Base Chemistry.
|
|
|
| |
| |
|
| 14) The titration of acids with bases will yield soluble salts. |
|
|
| Correct! |
|
| |
|
| |
|
| 16) There are a variety of acids and bases. Scientists would call anything near pH 1 a... |
|
|
| Correct! |
|
| |
|
| 17) There are a variety of acids and bases. Scientists would call anything near pH 14 a ... |
|
|
| Correct! |
|
| |
|
| 18) The pH is a measure of oxygen ions (O+). |
|
|
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
Kinetic Molecular Theory
|
|
|
| |
Click on the picture above to get an overview of the Kinetic Molecular Theory.
|
|
|
| |
| |
|
| 20) Particles in a gas are small and the motion of those particles is rapid, constant, and random. |
|
|
| Correct! |
|
| |
|
| 21) Gases can be defined by the following statement: |
|
|
| Correct! |
|
| |
|
| 22) Gas molecules move in straight lines at different speeds. |
|
|
| Correct! |
|
| |
|
| 23) The average kinetic energy is directly related to the absolute temperature. |
|
|
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
Empirical Gas Laws
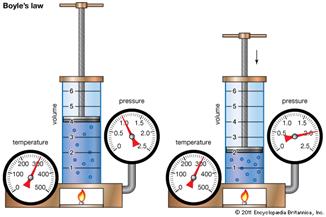
|
|
|
| |
Click on the picture above to get an overview of the Empirical Gas Laws.
|
|
|
| |
| |
|
| |
|
| |
|
| 27) As the temperature of a system increases, the pressure of the gases… |
|
|
| Incorrect |
|
| |
|
| 28) As the volume of a specific quantity of gas decreases, its pressure... |
|
|
| Correct! |
|
| |
|
| 29) Suppose you have two identical birthday balloons, with one filled with the gas helium and the other blown up by you. Assuming they’re both filled to the same amount, these balloons will have the same number of molecules. |
|
|
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
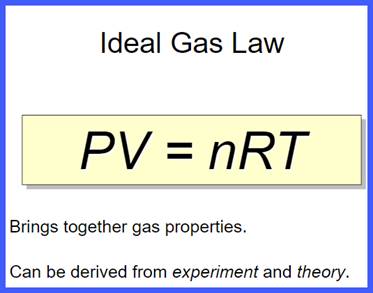
Ideal Gas Law
|
|
|
| |
Click on the picture above to get an overview of the Ideal Gas Laws.
|
|
|
| |
| 30) The Ideal Gas Law is a combination of Charles' Law and Boyle's Law. |
|
|
| Correct! |
|
| |
|
| |
|
| 32) Type the equation of the Ideal Gas Law. |
|
3994 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
| 33) A 4.0-liter container has two gases inside, neon and argon. It is known that at 18 °C, the total pressure of the combined gases is 0.850 atm. If it is known that there are 0.100 moles of neon in the container, how many moles of argon is in the container?
Calculator |
|
|
| Incorrect |
|
| |
|
| 34) How many moles of oxygen must be placed in a 3.00-liter container in order to exert a pressure of 2.00 atmospheres at 25 °C?
Calculator |
|
|
| Correct! |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
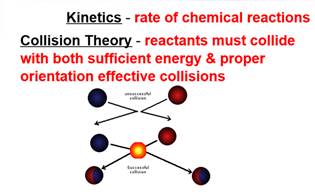
Kinetics
|
|
|
| |
Click on the picture above to get an overview of Kinetics.
|
|
|
| |
| |
|
| 36) The rate of reaction will increase with an increase in temperature. |
|
|
| Correct! |
|
| |
|
| 37) The rate of reaction will increase with a decrease in concentration. |
|
|
| Correct! |
|
| |
|
| 38) Catalysts increase the rate of a chemical reaction by what means? |
|
|
| Incorrect |
|
| |
|
| 39) In chemical reactions, electricity, a spark, and sunlight are all ways in which _______________ may be provided. |
|
|
| Incorrect |
|
| |
|
| 40) Which of the following will slow down the rate of a chemical reaction? |
|
|
| Incorrect |
|
| |
|
| |
|
...................................................................................................................................................................
|
|
|
| |
Equilibrium
Click on the link to watch an overview of Chemical Equilibrium.
Click Here
|
|
|
| |
| 42) Type the equation for equilibrium constant. |
|
3969 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
| |
|
| |
|
| |
|
| 46) Which of these choices can change the equilibrium constant? |
|
|
| Incorrect |
|
| |
|
| 47) What letter represents the equilibrium constant? |
|
|
| Correct! |
|
| |
|
| 48) The equilibrium constant references the position of the equilibrium. If you have a large equilibrium constant, your reaction would favor… |
|
|
| Correct! |
|
| |
|
.................................................................................................................................................................................................................................................................................
|
|
|
| |
49) Write a brief summary of your experience in this course in order to provide feedback that helps us to improve the course for future students.
|
|
3999 character(s) left
Your answer is too long. |
| Incorrect |
|
| |
|
50) Offline Activity
Write the following in the Log Entry.
- List all the dates and times that you prepared and studied for this quarterly exam
- In the description, write a brief summary on how you studied and prepared for this quarterly exam
|
|
| 05/30/2023 | 01:22 PM | 01:40 PM | unit 36 |
|
0 Hour(s) & 18 Minute(s)
|
| Excluded |
|
|
Attachments |
|