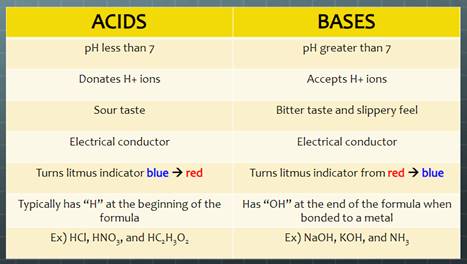
Acid-Base Chemistry
|
|
|
| |
| |
|
| |
|
Use the following terms to answer the following questions.
Word Bank
| solution |
solute |
| solvent |
concentration |
| molarity |
titration |
| volumetric |
quanitative |
| qualitative |
indicator |
|
|
|
| |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| 8) Strong acids and strong bases completely break apart into ions. |
|
|
| Correct! |
|
| |
|
| 9) Why do the products of a chemical reaction between a strong acid and a strong base have a pH of 7? |
|
3947 character(s) left
Your answer is too long. |
| Not Graded |
|
| |
|
| 10) What is the purpose of an indicator in the solution with the unknown concentration? |
|
|
| Correct! |
|
| |
|
| 11) What is the endpoint of a titration? |
|
|
| Correct! |
|
| |
|
| 12) Which solution usually goes in the burette? |
|
|
| Correct! |
|
| |
|
| 13) During a titration, _____ is carefully added to _______. |
|
|
| Correct! |
|
| |
|
How are titrations analyzed?
|
|
|
| |
| 14) A titration curve is a graphical representation of changes in pH over time. |
|
|
| Correct! |
|
| |
|
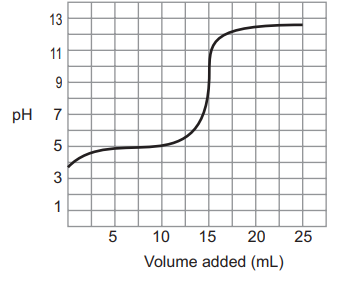
| Use the following graph below to answer the following questions. |
 |
|
| |
| 15) Before the titration began, what was the pH of the solution? |
|
|
| Correct! |
|
| |
|
| 16) At the equivalence point of the titration, what was the pH of the solution? |
|
|
| Correct! |
|
| |
|
| 17) At the endpoint of the titration, what was the pH of the solution? |
|
|
| Correct! |
|
| |
|
| 18) What volume of the titrant was added to reach the equivalence point? |
|
|
| Not Graded |
|
| |
|
| 19) What is the possible identity of the analyte? |
|
|
| Correct! |
|
| |
|
| 20) What is the possible identity of the titrant? |
|
|
| Correct! |
|
| |
|
| |
|
| 22) What is the possible identity of the substance that caused the endpoint? |
|
|
| Not Graded |
|
| |
|
Mathematical Calculations of Concentration
|
|
|
| |
| 23) What is the purpose of a titration experiment? |
|
|
| Correct! |
|
| |
|
| |
|
| |
|
| 26) What is the molar ratio between HCl and NaOH according to the balanced equation below?
HCl + NaOH --> H2O + NaCl |
|
|
| Correct! |
|
| |
|
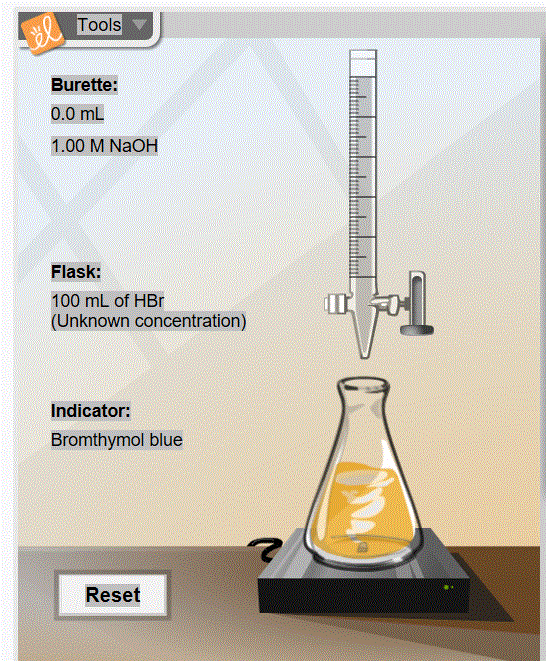
ChemLab: Titration
|
 |
|
| |
| 27) In a titration, the titrant is the substance that is placed in the burette and added to the analyte in the flask. |
|
|
| Correct! |
|
| |
|
| 28) In a titration, the analyte is a substance of unknown composition or concentration that is placed in a flask. |
|
|
| Correct! |
|
| |
|
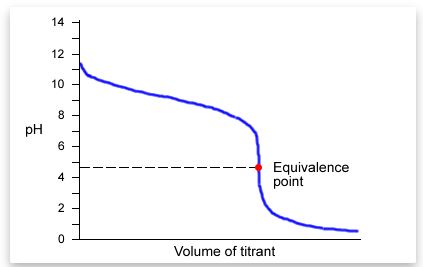
| 29) Which type of titration is shown by the titration curve below? |
 |
|
| Correct! |
|
| |
|
30) ChemLab: Titration
Student Exploration Lab Sheet
Click on the Resource tab for lab sheet, complete log entry and attach the document.
|
|
| No offline activities found |
0 Hour(s) & 0 Minute(s)
|
| Excluded |
|
|
Attachments |
|