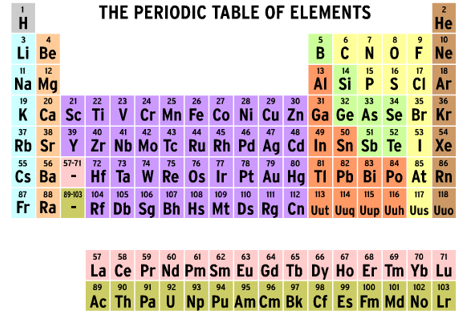
The Periodic Table
|
 |
|
| |
| 1) The periodic table of elements is the tabular arrangement of the elements in order of ________________ atomic number. |
|
|
| Correct! |
|
| |
|
| |
|
| 3) As you move from in the periodic table from left to right, we notice that the atomic number increases ... |
|
|
| Correct! |
|
| |
|
| 4) Which of the following is a true statement? |
|
|
| Correct! |
|
| |
|
| 5) Look at the Hydrogen element below.
What is the name of 1 and what does it indicate? |
 |
3925 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
What are the parts of the Periodic Table?
|
|
|
| |
| 6) What does the period of the periodic table tell you about the element? |
|
|
| Correct! |
|
| |
|
| 7) How does the period number relate to the number of shells an atom needs? |
|
|
| Correct! |
|
| |
|
| 8) All the elements in group 1 have the same amount of protons in their outside shell. |
|
|
| Incorrect |
|
| |
|
| |
|
| |
|
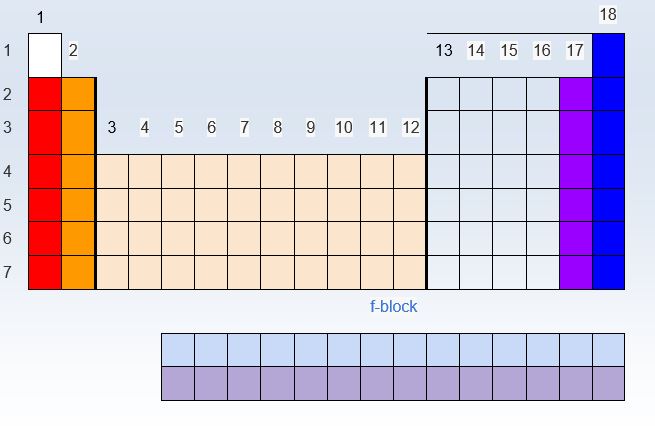
What are the named groups of elements on the Periodic Table?
|
 |
|
| |
| 11) What is the family name of the group 1A elements? |
|
|
| Correct! |
|
| |
|
| |
|
| 13) The f - block is part of the transition metal family, but because their final electrons enter an f-sublevel, they are denoted as .... |
|
|
| Correct! |
|
| |
|
14) BrainPOP Activity #1
Complete your log entry and attach your Activity #1 worksheet.
|
|
| No offline activities found |
0 Hour(s) & 0 Minute(s)
|
| Excluded |
|
|
Attachments |
|
| 15) What characteristics are shared by all alkali metals and alkaline earth metals? |
|
|
| Correct! |
|
| |
|
| 16) Noble gases are sometimes called "inert gases." What can you infer about the meaning of the word "inert" in chemistry? |
|
|
| Correct! |
|
| |
|
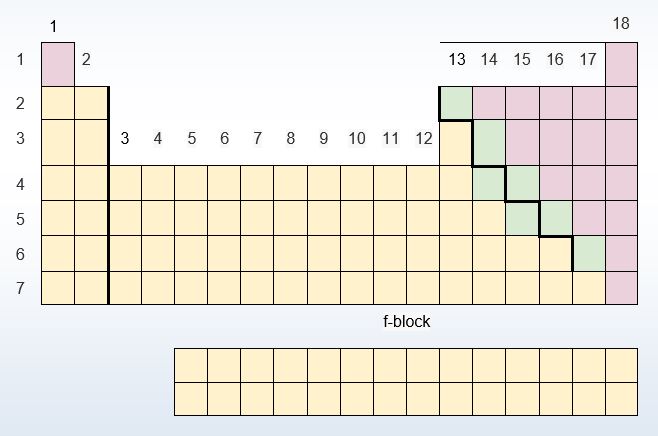
What are the general types of Elements on the Periodic Table?
|
 |
|
| |
| 17) List the three general types of Elements on the Periodic Table. |
|
3971 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
| 18) Briefly describe one of the general types of Elements on the Periodic Table. |
|
3661 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
19) BrainPOP Activity #2
Complete the log entry and attach your Activity #2 worksheet.
|
|
| No offline activities found |
| 0 Hour(s) & 0 Minute(s) |
| Excluded |
|
|
Attachments |
|
| 20) What can you infer from the fact that metals are good conductors of electricity? |
|
|
| Correct! |
|
| |
|
| 21) Elements with positive valences usually _________ electrons. |
|
|
| Correct! |
|
| |
|
| |
| 22) The periodic law states that the properties of elements repeat themselves throughout the periodic table. |
|
|
| Correct! |
|
| |
|
| 23) What are the outermost electrons called? |
|
|
| Correct! |
|
| |
|
| 24) Which of the following list of elements is arranged from smallest to the largest atomic radius?
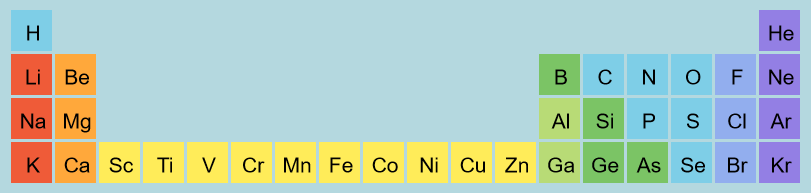
|
|
|
| Correct! |
|
| |
|
| 25) Which of the following groups is characterized by both a high ionization energy and a low electron affinity?
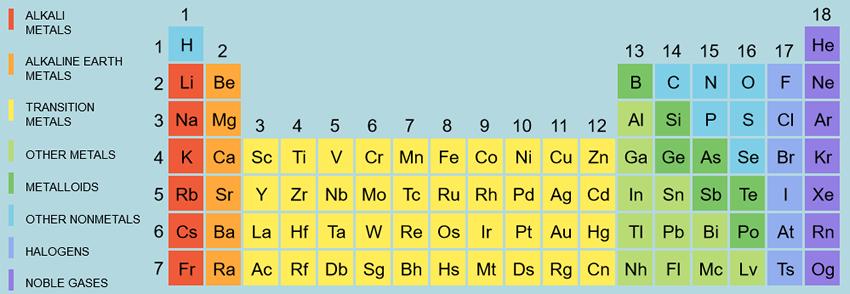
|
|
|
| Correct! |
|
| |
|
| 26) Extended Learning
Watch the following video, then write a five-sentence paragraph summarizing the video.
|
|
20000 character(s) left
Your answer is too long. |
| Not Graded |
|
|
Attachments |
|
27) ChemLab: Periodic Trends
Enter the following information in the Add a Log Entry:
- Date completed
- Start Time and End Time
- Description: Attach the completed Student Exploration Lab Sheet (click on Resource tab for a copy)
|
|
| No offline activities found |
0 Hour(s) & 0 Minute(s)
|
| Not Graded |
|
|
Attachments |
|