| |
| |
|
| |
|
| |
|
4) ChemThink! Covalent Bond Tutorial
Attach your ChemThink Guide to this question and complete your log entry.
|
|
| No offline activities found |
0 Hour(s) & 0 Minute(s)
|
| Excluded |
|
|
Attachments |
|
| 5) A covalent bond forms when ... |
|
|
| Incorrect |
| (D) is correct. |
|
| |
|
| 6) Which formula matches: diphosphorus pentoxide
|
|
|
| Correct! |
|
| |
|
| |
|
| 8) How many electrons are the atoms pictured below sharing? |
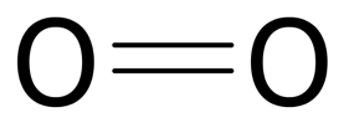 |
|
| Correct! |
|
| |
|
| 9) In a covalent bond, the atoms are held together by ... |
|
|
| Correct! |
|
| |
|
| 10) How many electrons are the atoms pictured below sharing? |
 |
|
| Correct! |
|
| |
|
| 11) How many electrons are the atoms pictured below sharing? |
 |
|
| Correct! |
|
| |
|
| 12) What is the name of the following formula: CS2
|
|
|
| Correct! |
|
| |
|
| |
| 13) What is the electronegativity difference between Carbon and Oxygen? |
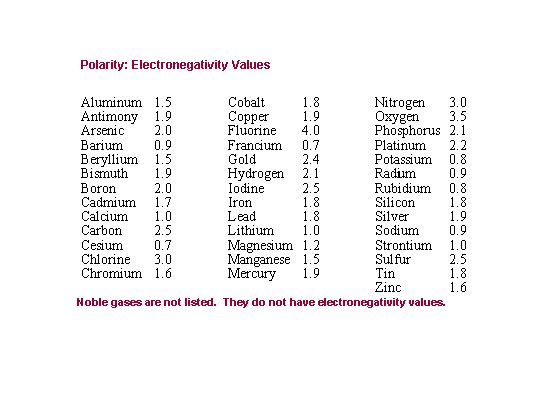 |
|
| Correct! |
|
| |
|
| 14) What is the electronegativity difference between radium and bromine, if bromine's electronegativity is 2.8? |
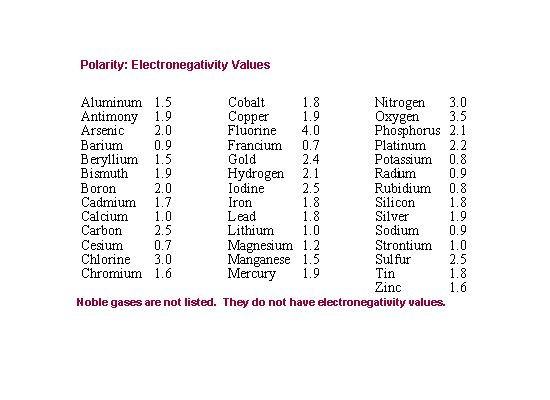 |
|
| Correct! |
|
| |
|
| 15) Atoms with low electronegativity will strongly attract electrons. |
|
|
| Correct! |
|
| |
|
| 16) Why will Carbon and Hydrogen share electrons equally? |
|
3958 character(s) left
Your answer is too long. |
| Correct! |
|
| |
|
| |
| 17) Covalent bonds occur between which type of atoms? |
|
|
| Correct! |
|
| |
|
| |
|
| |
|
| 20) Covalent bonds result from the sharing of electrons. |
|
|
| Correct! |
|
| |
|
| 21) When two pairs of electrons are shared, a ______ covalent bond is formed. |
|
|
| Correct! |
|
| |
|
| 22) Covalent bonds tend to be stronger than ionic bonds. |
|
|
| Correct! |
|
| |
|
Covalent Bonds
Choose a substance, and then move electrons between atoms to form covalent bonds and build molecules. Observe the orbits of shared electrons in single, double, and triple covalent bonds. Compare the completed molecules to the corresponding Lewis diagrams.
|
|
|
| |
| 23) In this model of a molecule of ammonia, NH3, how many covalent bonds are represented?
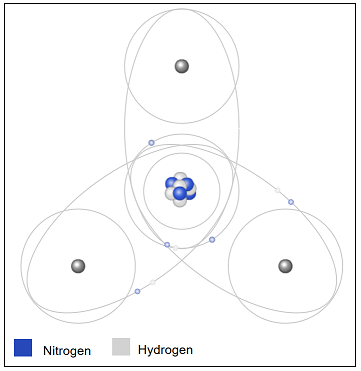
|
|
|
| Correct! |
|
| |
|
| 24) How many covalent bonds are there in one molecule of carbon dioxide, CO2?
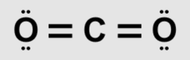
|
|
|
| Correct! |
|
| |
|
| 25) Extended Learning
Watch the following video, then write a five-sentence paragraph summarizing the video.
|
|
19962 character(s) left
Your answer is too long. |
| Excluded |
|
|
Attachments |
|
26) ChemLab: Covalent Bonds
Student Exploration Lab Sheet
Click on the Unit Resource tab for the Student Exploration Lab Sheet, complete the log entry and attach the document.
|
|
| No offline activities found |
0 Hour(s) & 0 Minute(s)
|
| Excluded |
|
|
Attachments |
|