WATER AND WATER POLLUTION

Properties
of Water
Water, dihydrogen oxide, H2O, such a small,
simple molecule, yet it is vital for life.
Let’s start by looking at water at the molecular level. Water is made up of two hydrogen atoms and
one oxygen atom. These atoms are COVALENTLY BONDED, which means
electrons are shared between the atoms. Hydrogen
has one electron in its outer most shell and oxygen has six. Molecules want to be STABLE that means eight electrons total in the outer most
shell. Note: Now that both hydrogens are bonded with the oxygen there are
now eight electrons total.
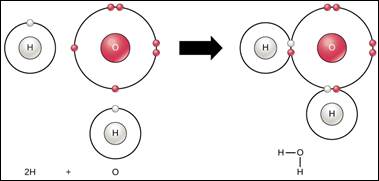
This bond causes the water molecules to be POLAR. There is an uneven charge. There is a negative charge near the oxygen
atom and a more positive charge near the hydrogen. The polarity is what makes other substances
dissolve in water. So when introduced to
another atom or molecule that is negatively charged it will be attracted to the
hydrogen atoms, a positively charged atom or molecule will be attracted to the
oxygen atoms. When more than one
molecule of water is involved they are attracted to each other by HYDROGEN BONDS (pictured below). The
hydrogen atoms are attracted to the oxygen atoms and vice versa.

These hydrogen bonds are responsible for many of
water’s unique characteristics. Ice
floats because the hydrogen bonds are further apart from each other in its
solid state. Water does tend to mix well
with most substances, however nonpolar substances repel water making them HYDROPHOBIC, this is because of the
hydrogen bonds. It is also the reason that droplets form and why there is a
meniscus in glasses. Water is attracted
to water, this is COHESION. Try this
at home, all you need is a penny and an eye dropper. Count how many drops of water you can fit on
the penny before the water spills. As
you drop water, you should notice the water stick together and eventually
create a “bubble” on the top of the penny.
This “bubble” is created by cohesion.
Cohesion is responsible for surface tension. This attraction of particles is what keeps
water bugs able to stay afloat. ADHESION is when water is attracted to
other substances. Cohesion is what forms
the water droplets; adhesion is what keeps the water droplets on your car or
drinking glass.

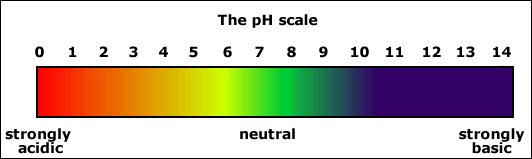
A chemical property of water is its pH. pH is the measure of how acidic or basic
a solution is. The scale measures from 0-14.
Solutions with a measurement of 0-6 are considered acidic and
measurements of 8-14 are basic. Water is
the magic number 7, neutral, it is neither acidic nor basic. The pH is an easy ways to measure the quality
of water. Another special property of
water is that it is the only natural substance that is found in all three
states of matter at the temperatures normally found on Earth. Water freezes at 32![]() and boils at 212
and boils at 212![]() .
.
The
Hydrologic Cycle
Now that we know how water works at a molecular level,
let’s look deeper on how it interacts with the environment. The HYDROLOGIC
CYCLE, also known at the water cycle, is the process of how water travels
as it goes from water vapor, to precipitation, then collects on lands and bodies
of water, and begins again.
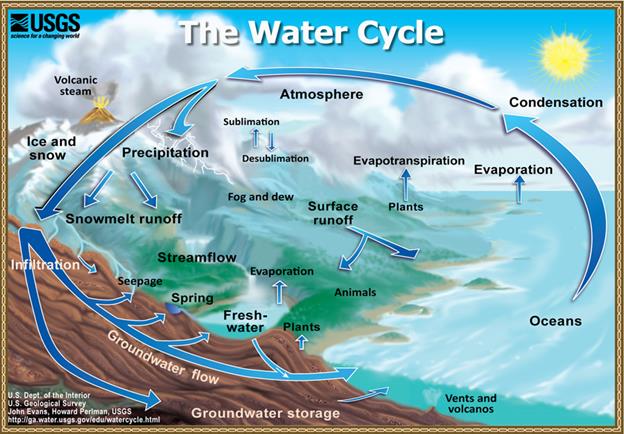
EVAPORATION
is
when water changes from a liquid state to a gas state, mostly from bodies of
waters such as lakes, rivers, and oceans.
Heat energy is needed to break the hydrogen bonds in order to separate
the water molecules. Water evaporation
does not just happen in bodies of water, it also comes from plants, and this is
called TRANSPIRATION. Once the water vapor rises, it starts to
cool. The cooling makes the vapor turn
back into a liquid; this process is known as CONDENSATION. Condensation
is what forms fog and clouds. The water
has to come back down to Earth. PRECIPITATION can be in the form of
rain, sleet, hail, or snow. From there
the water is COLLECTED by soaking
into the ground or running into bodies of water and the cycle continues.
Drinking
Water and Water Quality
Many people take for granted that water is always
available. Many countries are not so
fortunate. Even though the Earth is
mostly water only 3% is fresh water, and the majority of that water is frozen
in the cryosphere. The CRYOSPHERE is
the part of Earth’s water that is frozen, this includes, glaciers, ice caps,
and permafrost. In Ohio, there are two
main sources of drinking water; GROUND
WATER and SURFACE WATER. After
it rains the water either percolates into the ground (ground water) or flows
into lakes, rivers, streams, or reservoirs (surface water).
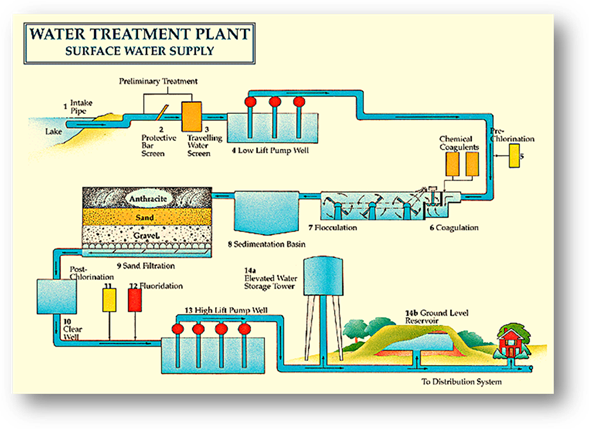
Here is the process, if you get your water through
surface water. First pumps are placed in
lakes, rivers, and reservoirs to collect water.
Through a pumping station and pipes, water travels to the water
treatment plant. The first step to start
cleaning the water is the use of coagulates.
COAGULATES cause particles to
“clump” to together. These “clumps” are
called FLOC. The process of getting rid of the floc is
called CLARIFICATION. By now most of the floc has been
removed. Water is then filtered through
layers of active carbon, sand, and gravel. The water is still not ready to be sent to
your house, there could still be microorganisms in it, so the last step is for
the water to be disinfected. DISINFECTION is when chemicals like
chlorine or ozone are added to kill off the microorganisms. The water is
finally clean enough to be considered drinking water and is sent through pipes
and a pumping station to your house.
Throughout the trip, water is continually tested. There are thousands of tests that are done;
some of the major ones include pH, E.coli, lead, fluorine, nitrate, and sulfide
levels. Watch the video below to see the
process for yourself.
Water
and You: The Water Treatment Process
Not all people use county or city water, some houses
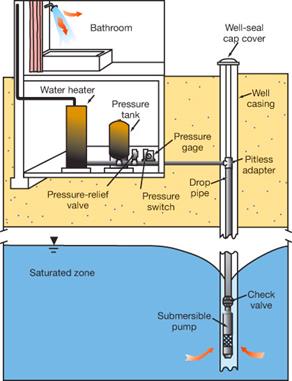
use a WELL SYSTEM. A well taps into a ground water storage area
called an AQUIFER. Wells can be shallow and may only have to go
twenty five feet down. However some many
need to be deeper in order to reach water. A pipe
is drilled down into the source of the water.
At the end of piping there is a pump that pulls in the water. The pump does not continuously bring it
water. The pressure tank and pressure
switch, control the water pressure in your house. This means that water is not just trickling
out of the facet when you wash your hands.
When the tank holding water inside your house starts to lose water the
pressure tank pushes the water through the pipes to get to the facet. As it is
doing that it is also pulling water from the reservoir. A benefit to having a well is that you do not
have to pay a monthly water bill.
However, you are responsible for any problems that may happen with the
system. In order to do maintenance on a
well, not all of it is located underground.
There is about a foot of pipe left above ground that allows for easy
access if necessary. Some disadvantages
to having a well is that if it does not rain for a while there may be less
water to pull from.
Water
Pollution and Commination
You probably noticed surface water is treated a
lot. That is because of all the
pollutants and toxins that get into it.
Ground water can also be polluted and filters are placed in the pumps to
reduce toxins. WATER POLLUTION is defined as the buildup of a substance so much
that is causes harm to plant and animal life.
The type of substances that enter our water systems is usually not the
problem. The problem occurs when so much
of a substance that the water cannot dilute it.
It is obvious that the overwhelming majority if water pollution comes
from humans and there are two major ways pollution can happen. When the location of the pollution can be pin
pointed to a location, it is called
POINT-SOURCE POLLUTION. Most of the
pollution is caused by many different sources; this is called NONPOINT-SOURCE POLLUTION. Usually the effects of pollution are most
felt near the source, however sometimes (seen with nuclear pollution) the
effects can be seen hundreds or thousands of miles away. Then it is can TRANSBOUNDARY POLLUTION. Sometimes pollution can be physically seen,
like after an oil spill. Other times the
chemicals and bacteria cannot be seen.
So how can we tell if water is contaminated? When remember when drinking water was
discussed? Water going through the pipes
was constantly collected and samples were sent away to get testes. Polluted water is chemically tested the same
way. There are also BIOLOGICAL INDICATORS. If
fish are not surviving in a lake or river, there is a good chance that it has
to do with poor water quality.
There are thousands of ways our water sources can get
polluted. Exhaust from a car can end up affecting
the ocean. Remember the water cycle. The
fumes of a car enter the atmosphere.
These fumes combine with the cooling water vapor. This will fall back to
the Earth. If the concentration of the
pollutant is high enough it can be considered acid rain. This rain can fall back into an ocean,
therefore containing it. So what you do
in Ohio may affect the water quality in a country across the world! Another way our waters are polluted is through
fertilizers. Farmers spray their crops
with nitrogen and phosphorus rich fertilizers that help plants grow. The fertilizers runoff into streams and
eventually make it into the bigger bodies of water. This excess of nutrients can cause ALGAL BLOOMS, more commonly known as RED TIDE. Algae and plankton as grow better in the
presence of nitrogen and phosphorus. The
over growth of these can cause the water to turn red. Red tide can lead to a DEAD ZONE. The algae and
plankton consume all the oxygen in the water.
Therefore nothing else has a chance to survive there.

About half of the ocean pollution is caused by sewage
and waste water. WASTE WATER is considered to be chemicals dumped down drains, and
untreated liquid waste from factories. A
lot of pollution and waste enters water from highway RUNOFF. Oil, brake fluid,
and other debris runs into streams and makes its way to the oceans, just like
the farmer’s chemical fertilizers. Waste
coming from the factories include polychlorinated biphenyls (PCBs), lead,
cadmium, and mercury. Fish that live in
mercury polluted water ingest the toxin then they contain the toxin as well. People eating these fish can get mercury
poisoning. Factories and industries will
also produce thermal pollution. Thermal
pollution is when water is returned to where it came from either warmer or
colder than the normal temperature. Aquatic
life cannot adapt fast enough to survive in the new temperature of water.

Chemical and oil spills are not the only culprit to
polluting the water. Shore lines can be
lined with trash, plastic being the most common. Most plastics are not biodegradable, so they
do not break up naturally in the environment.
Plastic can present a chocking hazard.
Marine life many also get tangled in plastic bags, drink holding rings,
and netting.

The link below is a video that shows all the major
types of water pollution.
What
is Water Pollution?
Water
Conservation and Clean up
With so many ways to contaminate the water, it is hard
to find a solution. Here are somethings
that have and continue to help. Make
people aware. Many take for granted the
clean water and do not notice the effects they may be contributing to. Laws that make factories limit their waste
water disposal, and the amount of chemical that can be released. Industries are usually fined for not obeying
these laws. We must also remember while
water covers 70% of the Earth, we must use it wisely, we use it faster than it
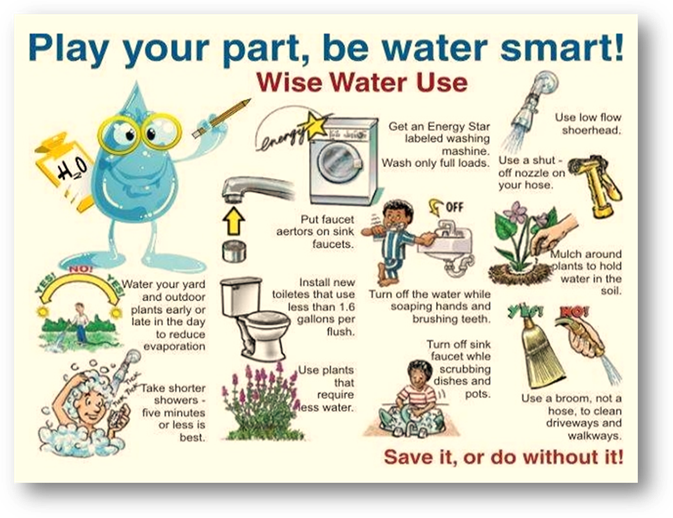
being replaced. The chart below shows your some ways you can conserve water.
 Now
answer question 1 through 22.
Now
answer question 1 through 22.