NUCLEAR REACTIONS

Unit Introduction
In Unit 12, you learned about nuclear energy. In this
unit, you will take a closer look—much closer, actually—at the atomic changes
involved in nuclear reactions. Recall the concepts of nuclear fission and
nuclear fusion. You will revisit these processes and gain a new understanding at
the molecular level. You will also explore the positive and negative effects of
radiation.
Nuclear Reactions
The study of radioactivity and nuclear reactions
requires a thorough understanding of the atomic nucleus. Since there can be
different isotopes (atoms whose nuclei have the same number of protons
but different numbers of neutrons) of the same element, a system was developed
to identify different forms of the same element.
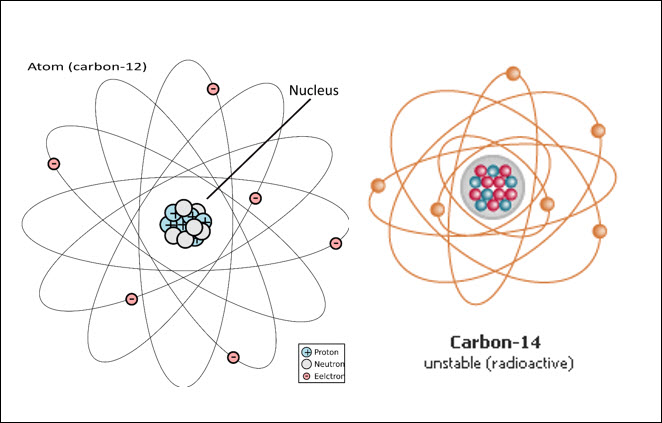
A nuclide is an atom that has a
specific atomic number and atomic mass. Carbon is an element that has more than
one isotope. One of the nuclides is carbon-12, and it is called carbon-12
because it has an atomic mass of 12. You will notice that the nuclide number (12)
and atomic mass (12) are the same. Carbon-12 is a stable nuclide, meaning it is
not radioactive.
What makes some nuclides stable, while others are
radioactive? The stable nuclides generally have the same number of protons and
neutrons. Carbon-12 has 6 protons and 6 neutrons making it a stable form of
carbon.
Another factor that makes an atom stable is the
balance of attractive nuclear forces and repulsive electrical forces. Nuclear
forces are the attractive forces between protons and neutrons in the
nucleus of an atom. Electrical forces are the repulsive forces among protons in the
nucleus.
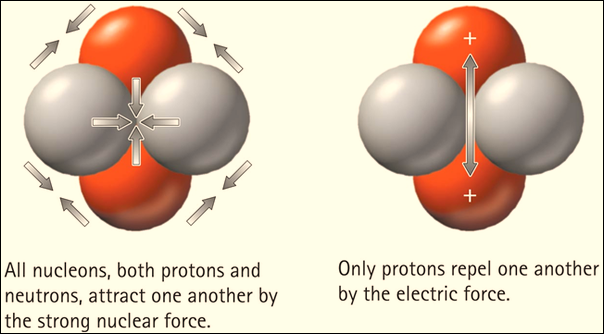
Nuclear forces are weak across long distances, but
within the short distances between protons and neutrons in the nucleus, they
are quite strong in holding the nucleus together. In a stable nuclide, there is
a balance between nuclear forces and electrical forces. When these forces are
unbalanced in an atom’s nucleus, this is known as a radioactive nuclide.
Another form of carbon is carbon-14, an isotope of
carbon, which has 8 neutrons and 6 protons. The number of protons and neutrons is
not equal, and neither are the nuclear and electrical forces in the nucleus of
a carbon-14 atom. This leads to an unstable nuclide which is radioactive.
Watch
the following video, which gives more information about nuclear stability.
Radioactive Decay
Nuclear
reactions are reactions of matter that involve
changes to the nucleus and release a great amount of energy. When an unstable
radioactive isotope breaks down over time, its nucleus changes, which results
in the formation of a more stable substance. This change or breakdown over time
is known as radioactive decay.
Now, watch the following video clip on types of
radioactive decay. Complete the guided notes as you watch. Submit your
completed work as question #15 in the assessment portion of the unit.
Printable: TYPES
OF DECAY VIDEO NOTES
Radioactive decay of a radioisotope over time can be
graphed. The amount of time it takes for half of a sample of a certain
radioisotope to break down into a more stable element is known as its half-life.
A half-life is unique and constant for each radioisotope. For example, the
half-life of uranium is different from the half-life of carbon-14. When you
graph the radioactive decay across several half-lives, you see a certain shape
on the graph. The following image shows the classic pattern of radioactive decay
on a half-life graph. The blue line represents the radioisotope, or “parent”
material, and the red line represents the more stable element, or “daughter”
material.
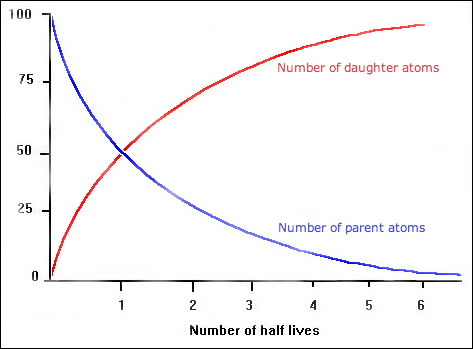
When a radioactive substance undergoes radioactive
decay, will the amount of the radioisotope ever reach zero? The answer is no.
That is because it is breaking down by half, over each half life. Half of 100
is 50, half of 50 is 25, and so on. Half of any number is never zero. The
amount will get smaller and smaller and approach zero, but never be zero.
The following article is an excellent resource which
has more information about radioactive decay and half-lives.
Once you’ve read the last article, watch this video
clip which shows you how to plot half-lives on a rate of decay graph. As you
watch the video clip, construct your own decay graph along with the video
narrator. Submit your work as question #16 in the assessment portion of the
unit.
Radioactive Dating
Half-life values of certain radioisotopes are used in radioactive
dating. Radioactive dating is used to find the age of fossils and rock
in Earth’s layers. Carbon-14 is a radioisotope found in living things. It has a
half-life of 5,370 years. If a fossil has half as much carbon-14 as a new bone,
for instance, an age of 5,370 years can be assigned to the fossil, since one
half life has passed.
Lab: Simulating Radioactivity
Complete Part I of the following SAS activity to
simulate radioactivity. The directions say to use pennies, but you can choose
whether you use pennies, M&M’s or Skittles. (The key here is having a
two-sided object to count—since the candies have one printed side and one plain
side, just as a coin has heads and tails, they will all work for this
activity.) If you don’t have 100 pennies/candies, start with another quantity
you do have, such as 50. You do not need to complete Part II. Complete pages 1,
2, and the top half of page 3 of the attached document and submit your work as question
#17 in the assessment portion of the unit.
Printable: SIMULATING
RADIOACTIVITY DOCUMENT
Nuclear Fission and Nuclear Fusion
As you recall from Unit 12, nuclear fission and
nuclear fusion are both types of nuclear reactions that release great amounts
of energy. Fission involves the splitting of large nuclei into smaller nuclei.
Fission is how nuclear power plants produce energy. Fusion is the joining of
smaller nuclei to create larger nuclei. This is how all the elements beyond
helium were created.
 Radiation and Its Applications
Radiation and Its Applications
Radiation has many positive applications, as well as
many negative consequences. Whether its use is considered positive or negative
largely depends on the amount of radiation that is used. It can be deadly if
exposure occurs in excessive amounts. The degree of damage caused by radiation
depends on many factors – dose, dose rate, type of radiation, the part of the
body exposed, age and health. For example, embryos, including the human fetus,
are particularly sensitive to radiation damage.
Radioactive substances can be placed in the soil
surrounding a plant and traced with a Geiger counter throughout the plant as
the plant absorbs minerals from the soil. Scientists can use this technique to
trace an element’s path through plants and animals to better understand where
nutrients go and how they are utilized by plants and animals.
Radiation can be used beneficially to kill cancer
cells. Radiation can also be used to detect tumors and diseased organs. Another
application is to sterilize medical equipment.
X-ray images of bones and teeth are produced using
radiation.

There are many industrial uses for radiation, as well
as harnessing energy of nuclear fuel in nuclear power plants.

The use of radioactive products can be very dangerous,
such as in a nuclear energy plant failure or explosion, or in the use of
nuclear weapons. Radiation has the power to destroy as well as to heal.
Now, read the following articles on the risks and benefits
of radiation.
https://hps.org/hpspublications/articles/risk-benefitinfosheet.html
Risks and Benefits of Radiation | American Scientist
QUIZLET VOCABULARY
Optional Extension: For additional information on the risks of radiation, read this thought-provoking excerpt of "The Radium Girls" by Kate Moore. It is a free Kindle download on Amazon.
https://www.amazon.com/Radium-Girls-Extended-Excerpt-ebook/dp/B06XB1WB7S/ref=sr_1_1?s=digital-text&ie=UTF8&qid=1494520380&sr=1-1&keywords=the+radium+girls+excerpt
 Now answer questions 1 through 17.
Now answer questions 1 through 17.

