CHEMICAL REACTIONS
![j0287065[1]](SCIPSU27_Chemical_Reactions_a_image003.png)
Unit Introduction
The purpose of this unit
is to allow you to investigate chemical reactions and learn how to write and
balance chemical equations.
Chemical Reactions
A chemical reaction occurs
when two or more substances interact in a way that produces new substances with
characteristics that are different from those of the individual components.
When a chemical reaction happens, the law of conservation of mass is
obeyed. That means that matter is not created or destroyed in a reaction, but
is conserved.
This video provides an
excellent foundation of knowledge on chemical reactions:
How do you know when a
chemical reaction has taken place? There are a few types of evidence you can
look for to know whether one has occurred.
|
EVIDENCE OF
CHEMICAL REACTION |
|
Color change;
for example, iron rusts and turns from a gray to a brown color
|
|
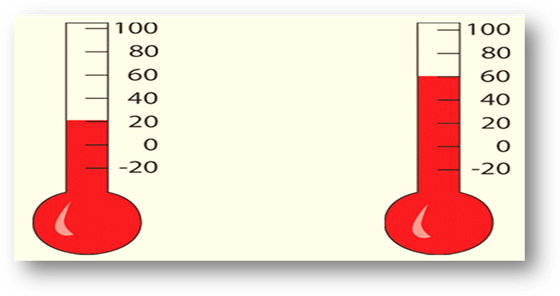
Temperature change; reactions will either take in or
give off heat energy, which affects the temperature of the substances
|
|
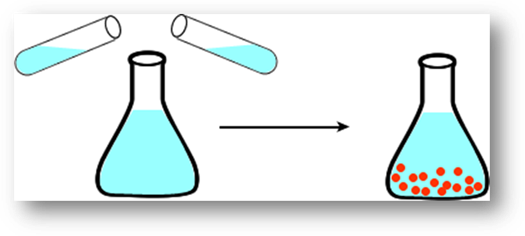
Formation of a precipitate; this is when a solid
forms in a liquid solution
|
|
Formation of a gas; example is the fizz produced
from dropping Alka-Seltzer in water
|
Energy can either be
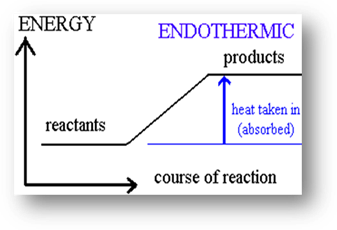
absorbed or released in a chemical reaction. An endothermic reaction
occurs when energy is absorbed as a result of the reaction. The prefix endo-
means into, and heat is taken in or absorbed in an endothermic reaction. The
temperature is perceived to be cooler at the end of the reaction than it was in
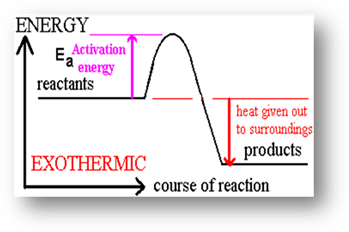
the beginning. An exothermic reaction occurs when energy is released because of
the reaction. The prefix exo- means outside, and heat
is given off during an exothermic reaction. The temperature at the end is
higher than it was before the reaction took place.
|
Endothermic
Reaction |
Exothermic
Reaction |
|
|
|
Chemical Equations
In previous units, you have
learned how to identify and write formulas for compounds. The next step is the
writing of a chemical equation. The
function of a chemical equation is to show changes that occur during a chemical
reaction. There are two ways to write a chemical
equation. The first is a word equation
that simply uses the words for the elements and compounds involved. An example of a chemical equation with words
would be “hydrogen plus oxygen yields water.”
The second type of chemical equation uses the symbols of those elements
and compounds involved rather than the words. An example of a chemical equation
with symbols would be 2H2 + O2 à 2H2O.
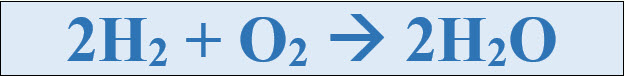
When a reaction occurs,
there are two types of substances: reactants and products. The substances to
the left of the arrow are called the reactants. Reactants are the elements and compounds that
must interact for the reaction to take place.
The elements and compounds to the right of the arrow are called the products. Products are the new substances produced in a
chemical reaction. The reaction happens
in one direction, which is indicated by the arrow. The arrow separates the
reactants and products and is read as “yields.”
So, the previous example would read “hydrogen plus oxygen yields water.”
Here are some basic
guidelines for writing chemical equations:
![]() Writing Chemical Equations (00:44)
Writing Chemical Equations (00:44)
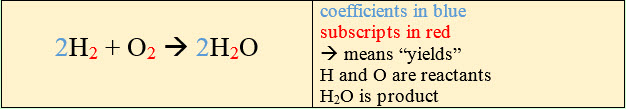
A number that precedes (or
comes before) the symbol or formula for an element or compound is called a coefficient.
The coefficient is distributed throughout the entire molecule. In the example above,
you have a coefficient of 2 preceding H2. This means that you have 2
H2 molecules. In the example you also see a coefficient of 2
preceding H2O. This means that you have 2 molecules of H2O.
The coefficients can be manipulated to balance the equation.
A subscript is a number
that follows an element symbol and is written in a somewhat lower position. The
subscript indicates the number of atoms for just the element it follows. So, in
H2O, the subscript of 2 indicates that there are 2 H atoms in the
molecule, but only 1 O atom. The subscripts cannot be manipulated to balance
the equation.
A coefficient and a
subscript are multiplied to get the total number of atoms of that element. For
example, 2SO4 has 2 sulfur atoms (coefficient 2 multiplied by a
single S atom) and 8 oxygen atoms (coefficient 2 multiplied by subscript 4).
Here is a pictorial
representation:
One SO4 molecule:
2SO4,
or two SO4 molecules:


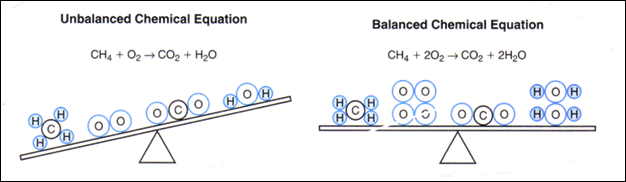
Balancing Chemical Equations
As mentioned previously,
when a chemical reaction occurs, the law of conservation of mass must be
obeyed. The way this is indicated in chemistry is to balance the chemical
equation at the molecular level. The number of atoms of each element in the
reactants must equal the number of atoms of each element in the products to
show the balance. Follow these steps to properly balance chemical equations.
1.
Write out the
formulas for all substances involved in the reaction.
![]()
2.
Count the number
of atoms of each element on both sides of the reaction.
![]()
3.
Do the numbers
balance? Yes = you are done! No = move on to step 4.
Ex: The number of carbon atoms on each side are equal,
but the number of iron and oxygen atoms on each side are different.
4.
Add coefficients
to make the quantity of atoms of each element equal on both sides of the
reaction. Note that the red coefficients below apply to the whole molecule that
comes after, and the coefficients and subscripts get multiplied.
![]()
5.
Double check your
numbers to make sure the equation is balanced.
![]()
Watch the following video
clip for additional explanation on how to balance chemical equations.
Now, try a few practice
problems to test your ability to balance equations. Keep going until you get 5
right in a row.
Here is even more
practice. Play the game to see if you have mastered the skill of balancing
equations.
QUIZLET VOCABULARY