NOMENCLATURE OF IONS AND COMPOUNDS
Unit Introduction
You have spent the past
few units learning about elements, ions, and chemical bonds. The purpose of
this unit is to introduce you to the system of naming ions, ionic compounds,
and covalent compounds.
Monatomic Ions
Recall from previous
units that ions are elements with a positive or negative charge due to the
imbalance of electrons. A monatomic ion is an ion of a single
atom. If you look at the periodic table, you can predict the charge of a
monoatomic ion based on the element’s group. Check out the periodic table below
to see these charges.
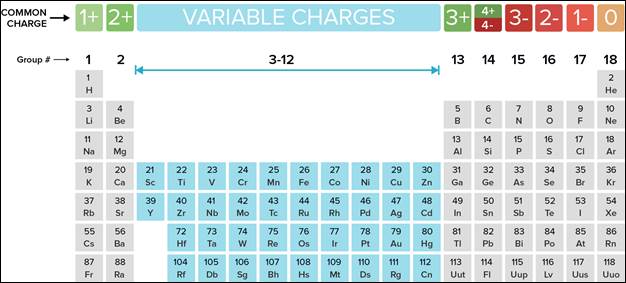
You can see that elements
in Group 1 have 1 valence electron, and when that electron is lost, the element
becomes a cation with a charge of 1+. When Group 2 elements ionize, they have a
charge of 2+. Transition metals in groups 3-12 have variable charges. Group 13
elements lose 3 electrons for a charge of 3+. The elements in group 14 can
either lose or gain electrons, which is why the
charges 4+ and 4- are both at the top of the chart. Group 15 elements gain 3
electrons to bring the charge to 3-. Elements of group 16 have 6 valence
electrons, and as they gain 2 more, the charge equals 2-. Group 17 elements
have 7 valence electrons and only need to gain 1 for a complete outer shell,
and the addition of 1 more electron makes the charge 1-. Finally, the elements
of group 18 have complete outer shells, do not commonly ionize, and have a
charge of 0.
The symbol for a
monatomic ion will be the element’s chemical symbol followed by the charge.
The chemical symbol is denoted by one or two letters. The first letter is
always capitalized and the second letter (if there is one) is always
lower-case. Here are some examples of monatomic ions and their abbreviations.
|
Element |
Symbol |
Charge |
Ion |
|
Aluminum |
Al |
3+ |
Al3+ |
|
Hydrogen |
H |
1+ |
H+ |
|
Chlorine |
Cl |
1- |
Cl- |
|
Oxygen |
O |
2- |
O2- |
|
Calcium |
Ca |
2+ |
Ca2+ |
When monatomic cations
are named, there are no special rules. If hydrogen is referenced, you can
simply call it hydrogen or a hydrogen ion. However, if the cation is
polyvalent, meaning it can have different charges, you need to name it
specifically with its charge. For example, iron or Fe can ionize in 2+ or 3+
forms. In this case, Fe2+ should be called “iron two plus” or “iron
two” and Fe3+ should be called “iron three plus” or “iron three, to
indicate the number of electrons lost and the ion’s charge.
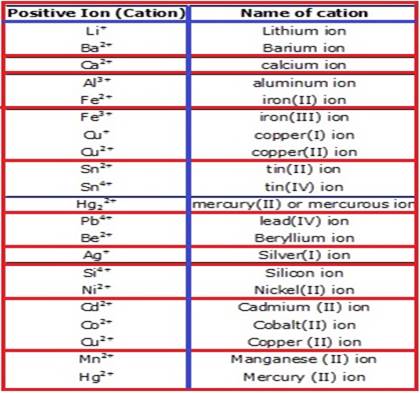
When monatomic anions are
named, usually the suffix -ide replaces the last part of the element’s name.
For example, a chlorine atom is called chloride, and an oxygen ion is called
oxide.
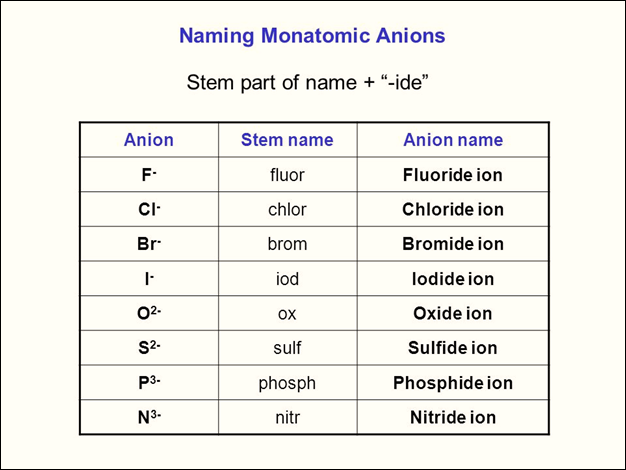
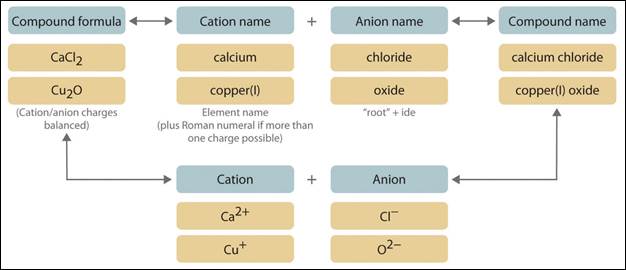
Ionic Compounds
Ionic compounds are formed when a cation and an anion form a chemical
bond through the transfer of electrons. When ionic compounds are named, there
are some rules that need to be followed:
·
name the cation
first, then the anion
·
ionic compounds
form with a net charge of zero
·
write ionic
compounds with the lowest possible integer value, ex: NaCl instead of Na3Cl3,
etc.
What would the name be
for the compound AlCl3? The cation is aluminum, and the anion is
chloride. Because aluminum has a 3+ charge, three chlorine atoms are needed to
each accept one of aluminum’s valence electrons, which is why chlorine is
followed by a subscript 3. One Al3+ ion plus three Cl-
ions will combine with a net charge of zero. The name of this compound, AlCl3,
is aluminum chloride.
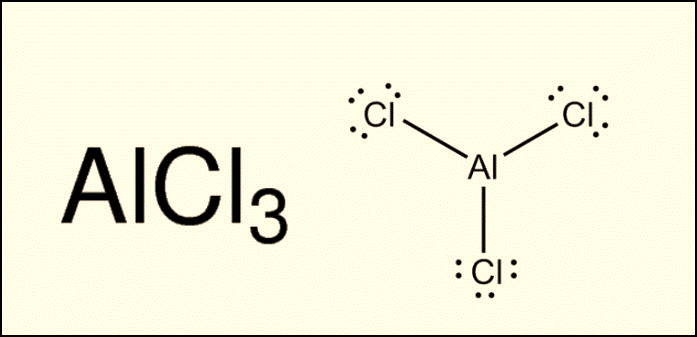
The next
image shows a few more examples of ionic compounds with the names and formulas
indicated, as well as an explanation of the transfer of electrons.

For more information on
naming monatomic ions and ionic compounds, please read the following resource
from Khan Academy. Answer the practice questions on the website as you read
through the lesson to check your understanding.
Watch the following video
clip about naming ions and ionic compounds:
Polyatomic Ions
A polyatomic ion is an ion
that contains more than one atom. It can be a diatomic molecule or a compound
molecule. The reason it is considered an ion is because it is charged, due to
an imbalance of electrons and protons. Reference the following site for more
information and a list of common polyatomic ions.

Binary Covalent
Compounds
A covalent compound is
formed when two nonmetals join in a chemical bond through the sharing of
electrons. The word binary means two. A binary covalent compound is made by
combining two elements. One nonmetal will act as the positive charge and
another nonmetal will act as the negative charge. The naming of covalent
compounds follows a set of rules:
·
write the name of
the first element
·
add a prefix to
the first element name (if more than 1) to indicate the number of atoms
·
write the root of
the second element and add “-ide” suffix
·
add a prefix to the second element name to indicate the
number of atoms.
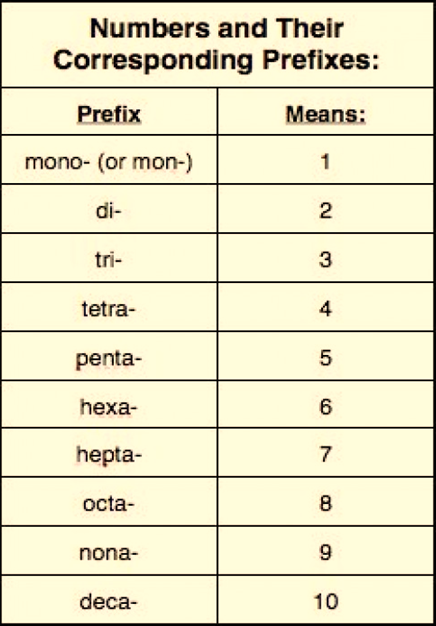
Here are a few examples
of covalent compounds with the formulas and names listed. Can you predict the
name of the last compound?

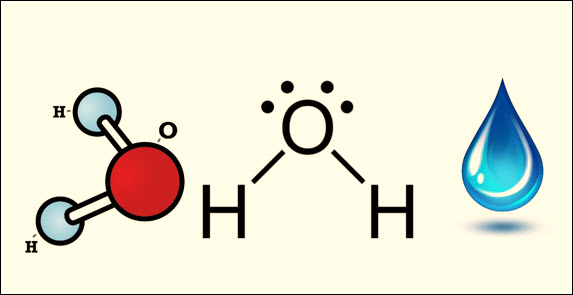
Follow the rules above to
give a name to chemical formula H2O.
Name of first element –
hydrogen
With prefix – dihydrogen
Name of second element –
oxide
With prefix – monoxide
Put it all together –
dihydrogen monoxide. What is this commonly known as? Water!
Often, binary covalent
compounds have common names in addition to the system names. Here is a table
showing examples of compounds with their system names and
common names. This table also shows how only two elements can combine in
different ratios to form several unique compounds.
|
FORMULA |
COMMON NAME |
SYSTEM NAME |
|
N2O |
nitrous oxide |
dinitrogen monoxide |
|
NO |
nitric oxide |
nitrogen monoxide |
|
N2O3 |
nitrous anhydride |
dinitrogen trioxide |
|
NO2 |
nitrogen dioxide |
nitrogen dioxide |
|
N2O4 |
nitrogen tetroxide |
dinitrogen tetroxide |
|
N2O5 |
nitric anhydride |
dinitrogen pentoxide |
|
NO3 |
nitrogen trioxide |
nitrogen trioxide |
QUIZLET VOCABULARY
