CHEMICAL BONDING AND COMPOUNDS
Unit Introduction
Matter exists as both
elements and compounds in nature. This unit’s purpose is to introduce you to
how elements can react to form different types of compounds.
What is a Compound?
A compound is a pure
substance that is made of more than one element. A compound is formed when two
or more elements react and form a chemical bond with one another. The
newly-formed compound has properties that are different from the properties of
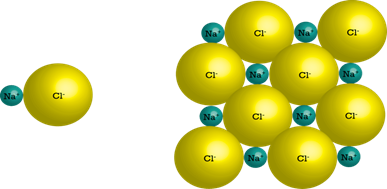
the elements it is composed of. For example, sodium (chemical symbol: Na) is a
soft, explosive metal and chlorine (chemical symbol: Cl) is a poisonous gas.
When they react to form a compound, sodium chloride (NaCl), it forms ordinary
table salt. It is neither explosive nor poisonous and is safe to eat.
The smallest piece of a
compound that still retains the compound’s properties is known as a molecule. A
molecule
is two or more atoms that have bonded together.
A compound cannot be
easily separated into its individual components because the elements are joined
by chemical bonds. These chemical bonds are so strong because of the sharing or
redistributing of electrons among atoms in the compound.
Video Clip: Compounds and Reactions
Watch the following video
clip to learn more about how elements react and form compounds. Then, complete
the post-test activity. Upload your document as question #12 in the assessment
portion of the unit.
Printable copy: COMPOUNDS AND REACTIONS POST TEST
Two Types of Bonds
There are two basic types
of chemical bonds. These bonds are determined by what happens to the atoms’
valence electrons when elements form compounds.
If electrons are shared
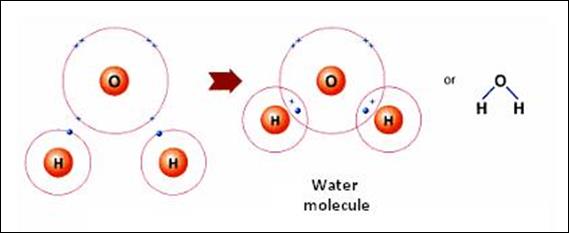
between two or more elements’ atoms, they form a covalent bond. Covalent
bonding often happens between two nonmetals. An example of a compound with a
covalent bond is water. A hydrogen atom has one valence electron and an oxygen
atom has 6 valence electrons. The chemical formula for water is H2O,
meaning there are 2 hydrogen atoms and 1 oxygen atom in a water molecule. So, a
total of 8 shared “valence” electrons form a complete shell when these three
atoms are bound together in a covalent bond.
Read the following
article to learn more about covalent bonding.
https://www.sciencedirect.com/topics/chemistry/covalent-bond
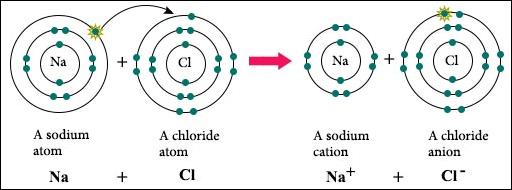
If electrons are
transferred from an atom of one element to an atom of another element, they
form an ionic bond. An ionic bond happens most often between metallic
cations (positive charge from losing 1-2 electrons) and nonmetallic anions
(negative charge from gaining 1-2 electrons. A common example of an ionic bond
is sodium chloride. Sodium has one valence electron, and chlorine has 7 valence
electrons. It takes much less energy for sodium to lose one electron to
chlorine than it would take for chlorine to lose 7 electrons. So, sodium gives
up 1 electron and becomes Na+. Chlorine takes that electron and becomes Cl-. As
a result of this electron transfer, a powerful ionic bond holds together the
NaCl molecule. Each end of this ionic compound has a charge because of the loss
or gain of electrons.
For more information on
ionic compounds, read the following article:
Compounds at the Molecular Level
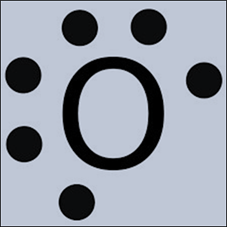
A Lewis dot structure shows
valence electron placement within an atom. You have seen examples of Lewis dot
structures, which show the electron configuration of one element’s atom, or
even the electron sharing or transfer when elements form compounds. The first energy level of an atom can hold a
maximum of two electrons, and if an atom or compound follows the octet rule,
every other energy level will hold a maximum of 8 electrons. Recall that
valence electrons in an atom are the “leftover” electrons in the outermost
energy level.
Read over the key
concepts of drawing the Lewis dot diagrams at the following website.
http://www.ausetute.com.au/lewisstr.html
Please watch the
following video to learn more guidelines of drawing Lewis dot structures.
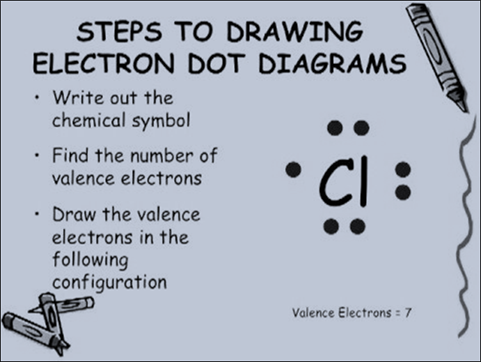
While there are no hard
and fast rules about where each dot must be placed in the Lewis dot diagrams,
typically you should place one dot in each location around the chemical symbol
(top, bottom, left, and right) before placing a second dot in those locations.
Since there are 8 electrons in a complete outer shell, there can be up to 8
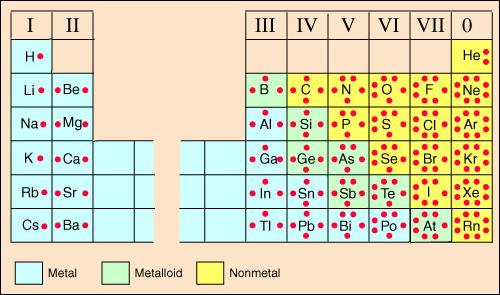
dots in a Lewis dot diagram. Refer to the chart below to see Lewis dot
structures for several elements on the periodic table.
Practice: Counting Valence Electrons
Now, visit the following
website and practice using the periodic table to answer questions about valence
electrons. Keep going until you answer 5 consecutive questions correctly.
QUIZLET VOCABULARY
