IONS AND ISOTOPES
Unit Introduction
To build upon what you
learned about atoms in the last unit, you will now investigate the result of
atoms gaining or losing subatomic particles and how that affects the identity
and characteristics of the atoms.
Atomic Number and Atomic Mass
Each element’s atoms has a different number of protons, as you may recall from the last unit. The number
of protons in an atom is indicated on the periodic table by the element’s atomic
number. The atomic number is also equal to the number of electrons in a
neutral atom. The number of protons and neutrons in the nucleus of an atom is
equal to the atom’s atomic mass. The atomic mass for each element is also indicated
on the periodic table.
atomic number = # of protons OR # of
electrons
atomic mass = # of protons PLUS # of
neutrons
Here are some examples of
periodic squares:
|
Element: |
Lithium |
Neon |
Titanium |
|
Periodic
Square: |
|
|
|
|
Atomic Number: |
3 |
10 |
22 |
|
Atomic Mass: |
6.941 |
20.1797 |
47.867 |
Isotopes
You may have noticed that
the atomic numbers are whole numbers, but often the atomic masses of elements
are decimal numbers. What does that mean, exactly? You know that the atomic
mass is equal to the number of protons and neutrons in an atom. But there aren’t
partial subatomic particles causing this number to be a decimal. Where is the
decimal coming from?
Sometimes, different
versions of an element can exist in nature (or in a lab-created setting). The
different versions have different numbers of neutrons in the nucleus. These
different versions of the element are called isotopes. An isotope is an atom of an
element with a different number of neutrons. The atomic mass of an element is
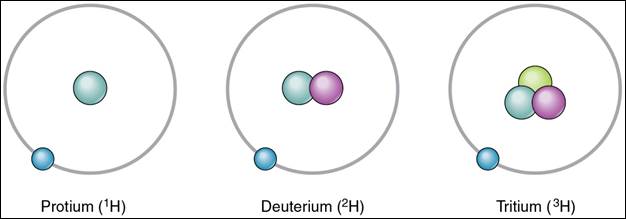
determined by averaging the isotopes according to their prevalence. For example,
the element hydrogen has 3 isotopes, including protium, deuterium, and tritium. Hydrogen has the atomic number 1, meaning hydrogen
has 1 proton and 1 electron. Protium has 0 neutrons, deuterium has 1 neutron
and tritium has 2 neutrons.
|
Element |
Isotope |
Protons |
Neutrons |
Electrons |
Mass |
|
Hydrogen |
Protium |
1 |
0 |
1 |
1 |
|
Hydrogen |
Deuterium |
1 |
1 |
1 |
2 |
|
Hydrogen |
Tritium |
1 |
2 |
1 |
3 |
The atomic mass is
calculated by averaging each isotope’s abundance and mass.

Hydrogen’s atomic number = 1
Average atomic mass = 1.00794
Based on hydrogen’s
atomic mass, which isotope do you think is the most common? Since the atomic
mass is 1.00794, which is very close to 1, the most common isotope must be
protium, which has zero neutrons.
Here is what each
hydrogen isotope’s atomic structure looks like:
Watch the following video
clip to learn more about atomic number, atomic mass, and isotopes:
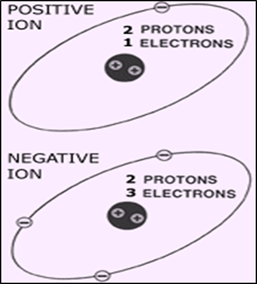
Ions
Atoms are electrically
neutral when they have the same number of protons and electrons. For example,
carbon’s atomic number is 6. This means that carbon has 6 protons. If a carbon
atom is electrically neutral, it must also have 6 electrons. What do you think
happens if a carbon atom has more or less than 6 electrons? If you think it
changes the electrical charge of the atom, then you are correct!
Imagine that a carbon
atom has 7 electrons. If that carbon atom has 6 protons and 7 electrons, there
are more negatively charged particles than positively charged ones. Therefore,
the charge of that carbon atom is -1. Now predict what the charge would be of a
carbon atom which has 6 protons but only 4 electrons. This particular carbon
atom would have more positive charge than negative charge. If you predicted
that the charge of this carbon atom is +2, then you are right.
When an atom has a
positive or negative electrical charge due to an imbalance of protons and
electrons, the atom is known as an ion.
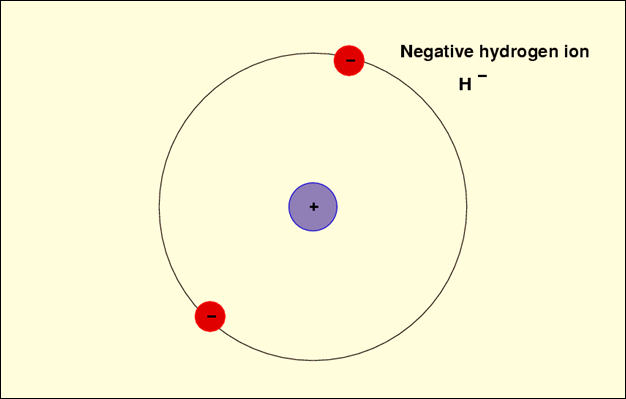
The following image shows
a hydrogen ion with a charge of -1. H, the chemical symbol for hydrogen, is
followed by a superscripted negative sign to show the charge.
There are two types of
ions that are defined by their electrical charges. A cation is an ion with a
positive (+) charge. Cations have less electrons than protons. An anion
is an ion with a negative (-) charge. Anions have more electrons than protons.
|
|
|
|
|
|
Valence Electrons
Remember the Bohr models
from the last unit? Also called the planetary model, the Bohr model of an atom
has energy levels known as electron shells where the electrons are drawn. The
electrons fill the shells closest to the nucleus first, and the electron shells
are populated from the center outward. If an element has a complete outer shell
of electrons, it is stable and doesn’t usually react with other elements. If
the outer shell of electrons is not full, these “leftover” electrons are known
as valence
electrons. The valence electrons are only the electrons in the outer
shell, furthest from the nucleus. The first electron shell only holds 2
electrons, and then the next shell holds eight. The third shell will also hold
eight. Most elements need 8 electrons in the outer shell in order to be stable.
If an element has less than 8 valence electrons, it is unstable and reactive
with other elements. An element will take electrons from other elements or lose
electrons to other elements in order to become more stable.
For example, sodium (Na)
has one valence electron. In the Bohr model below, you can see two electrons in
the first shell (full), eight electrons in the next shell (full), and one
electron in the valence shell. This makes sodium extremely reactive and
unstable.

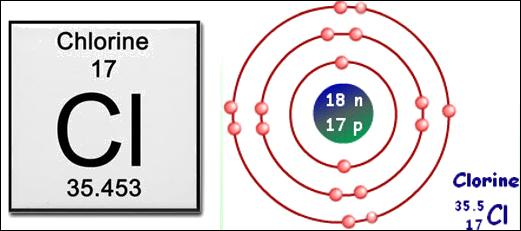
Chlorine (Cl) has seven
valence electrons. In the Bohr model below, you can see two electrons in the
first shell (full), eight electrons in the second shell (full), and seven
electrons in the valence shell. This makes chlorine extremely reactive and
unstable.
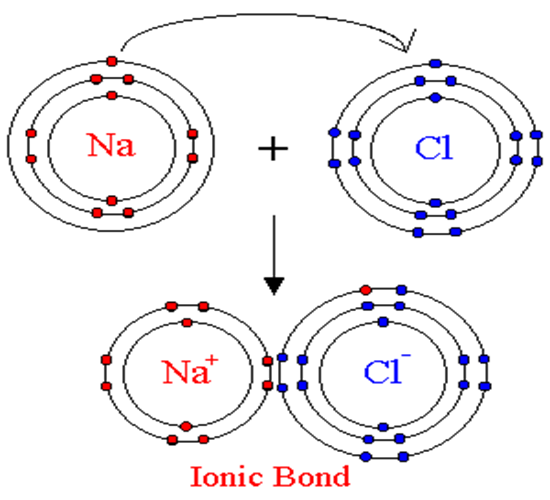
Since
sodium has one valence electron and chlorine has seven valence electrons,
sodium has the tendency to give up its one electron to chlorine. This is the
lowest energy way to make both elements more stable. That way, sodium has no
valence electrons and chlorine now has 8 electrons in the outer shell, meaning
it has no valence electrons either. Sodium becomes an ion with a positive
charge, or a cation. Chlorine becomes an ion with a negative charge, or an
anion. This “donation” of an electron creates a chemical bond between sodium
and chlorine to make the compound known as sodium chloride.
PhET Simulation: Build an Atom
If you are having problems using the simulation in the unit open up the simulation at
the following website and complete the activity. Submit your completed document
as question #12 in the assessment portion of the
unit.
https://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html
Printable: PhET BUILD AN
ATOM ACTIVITY
QUIZLET VOCABULARY