ATOMIC MODELS AND COMPONENTS OF THE
ATOM
![bd06978_[1]](SCIPSU22_Atomic_Atom_image002.png)
Unit Introduction
In this unit you will
learn all about the atom. First, explore the history of atomic models and
contributions of various scientists. Then, learn about the components of atoms.
Models of the Atom
In science, the smallest
particle that can exist and still have the properties of the element is called
an atom.
Science has developed many models throughout our scientific history to describe
or illustrate the makeup of an atom. Over time, technology has allowed
scientists to discover more details about the structure of atoms.
|
Scientist |
Time |
Background |
Summary |
Image of Model |
|
Democritus |
470-380 BC |
Greek philosopher; known as the father
of modern atomic thought |
matter is made of small particles called
“atomos” that cannot be broken down any further |
|
|
Dalton |
|
|
|
|
|
Thomson |
|
|
|
|
|
Rutherford |
|
|
|
|
|
Bohr |
|
|
|
|
|
Chadwick |
|
|
|
|
|
Modern |
|
|
|
|
The currently popular atomic
model is called the electron cloud model.
In the case of the atom, models are being used to describe or illustrate
something that is too small to see. We cannot see an atom and its components
with the naked eye, so a model allows us to conceptually grasp what an atom
looks like.
Atomic models of helium and carbon:
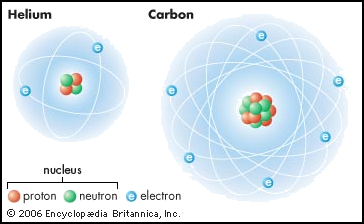
Atomic Components
Atoms are very tiny
particles that consist mostly of empty space. Atoms are made of even smaller
components, known collectively as subatomic particles. There are three main
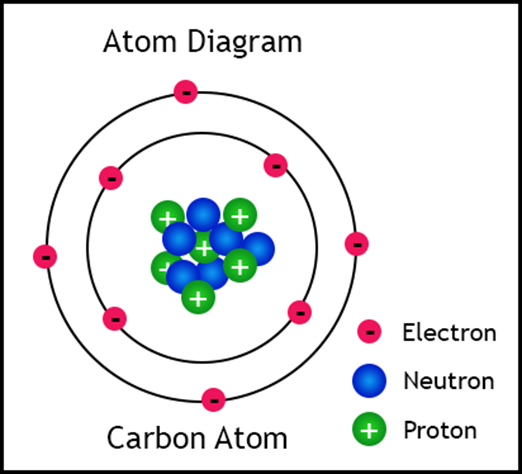
subatomic particles, known as protons, electrons, and neutrons. Protons
have a positive charge and they are found in the nucleus. Neutrons have a neutral
charge and they are also found in the nucleus. Since the nucleus has particles
of neutral and positive charges, the overall charge of the nucleus is positive.
Atoms are electrically neutral overall, which means that there needs to be an
area of negativity to balance out the positive nucleus. That negativity is
found in electrons. Electrons have a negative charge and they orbit around the
outside of the nucleus.
Watch the following video
clip to learn more about the atom and its components:
This video clip shows an
interesting perspective on how electrons move about inside the atom:
Atomic Mass
Protons and neutrons
contribute to the mass of the atom, but the mass of electrons is negligible
since they are so tiny. One proton has a mass of one atomic mass unit, or 1
AMU. One neutron also has a mass of 1 AMU. The mass of electrons is not considered
when calculating the total mass of the atom.
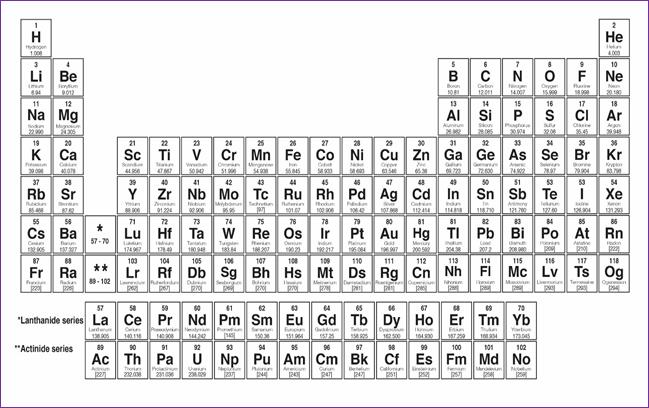
What makes an atom of one
element different from an atom of another element is the number of protons in
its nucleus, or its atomic number. The number of protons plus the number of
neutrons in its nucleus is known as its atomic mass. When you look at the
periodic table, such as the one below, the atomic number is at the top and the
atomic mass is indicated for each element at the bottom of the square.
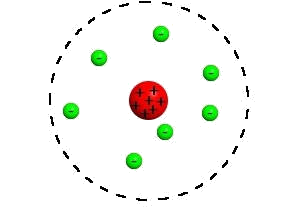
Rutherford’s Experiment
What we know about the
structure of the atom and the charged particles is due in large part to Ernest
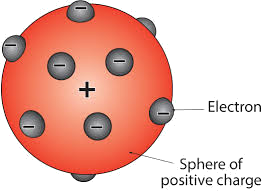
Rutherford’s gold foil experiment. Previously to his studies, the widely
accepted atomic model was Thomson’s raisin bun model, which hypothesized a
positively charged solid atom with negatively charged particles dispersed
throughout the atom. The raisin bun model Thomson developed looked like this:
Rutherford wanted to
expand knowledge of the atom, so he proposed that blasting alpha particles
through a thin sheet of gold foil would cause the particles to penetrate the
foil or possibly be deflected off to the side. What he found is that every once
in a while, particles bounced back. This led to his discovery of a tiny, but
massive, positively charged nucleus in the center of the atom, as well as
negatively charged electrons that circle the nucleus in empty space surrounding
the nucleus. His nuclear model of the atom looked like this:
The following video clip
will help to expand your knowledge and understanding of Rutherford’s gold foil
experiment. As you watch, create an annotated diagram that shows the experiment
with the information that Rutherford discovered by completing the experiment.
Scan or photograph your diagram and submit your work for question #13 in the
assessment portion of the unit.
PhET Simulation: Rutherford Scattering
Now, imagine that you are
conducting Rutherford’s gold foil experiment. Go to the following website to
interact with the simulation. Complete the attached document and submit your
work for question #14 in the assessment portion of the unit.
STUDENT PHET SIMULATION DOCUMENT
Quizlet Vocabulary
 Now answer
questions 1 through 14.
Now answer
questions 1 through 14.
