STATES OF MATTER AND PHASE CHANGES
Unit Introduction
In this unit, you will
explore the states of matter and the conditions needed for Phase changes in
matter.
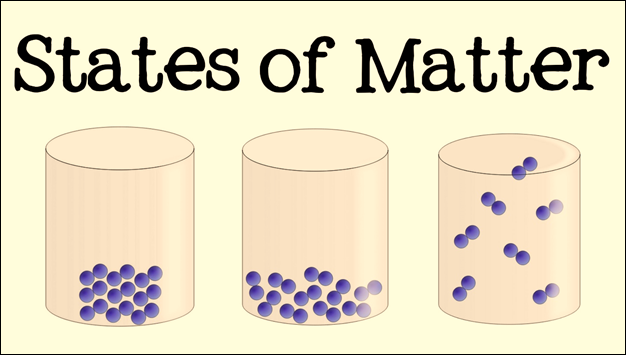
The States of Matter
Watch the following video
clip as an introduction to the states of matter:
https://www.brainpop.com/science/matterandchemistry/statesofmatter/
Then, take the review
quiz:
https://www.brainpop.com/science/matterandchemistry/statesofmatter/quiz/
There are three main
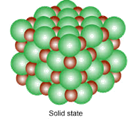
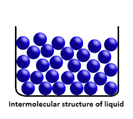
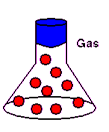
states of matter that are possible for all substances. These states are solid,
liquid, and gas. Each state of matter has its own unique characteristics.
|
State: |
Solid |
Liquid |
Gas |
|
Particle
Arrangement: |
packed tightly in regular formation |
not as close, particles flow amongst each other |
particles are far apart and fly around in all
directions |
|
Particle
Movement/Energy: |
slow vibration but particles mainly stay in place |
faster movement, more energy than solids |
fastest movement and highest energy among particles |
|
Shape: |
definite shape (holds its own shape) |
no definite shape (takes shape of its container) |
no definite shape |
|
Volume: |
definite volume |
definite volume |
no definite volume |
|
Image: |
|
|
|
Here is another diagram
to help you further visualize the similarities and differences among the states
of matter:
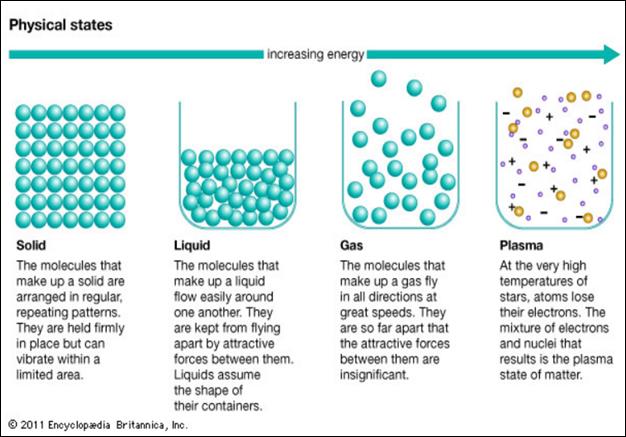
Plasma is a less common
state of matter that is found at extremely high temperatures produced by stars.
There is additional
information regarding the states of matter and an introduction to phase changes
in the following video clip. Pay close attention to the segment that starts
around 8:45. The narrator will draw a phase change diagram and explain what
conditions are needed for phase changes in matter.
Attach your completed
guided notes document to question #12 in the assessment portion of the unit.
Phase Changes
There is a common
misconception regarding temperature and phase changes. Many people believe that
increasing or decreasing temperatures are responsible for phase changes in
matter. While there is a temperature difference between solids and liquids or
liquids and gases, it is not a difference in temperature that results in a
phase change. Rather, it is the increase or decrease in the energy of the
particles that results in a phase change. Applying or removing heat does cause
a change in temperature, but it also causes a change in energy that is needed
for phase changes.
For example, water
freezes at 0 degrees Celsius but it also melts at 0 degrees Celsius. Anything
below zero exists as frozen water, or ice. Anything above zero up to 100
degrees Celsius exists as liquid water. At 100 degrees Celsius, water boils or
it condenses. Water above 100 degrees Celsius exists as water vapor (gas).
Since the melting point and the freezing point occur at the same temperature,
the change in energy is what determines whether the water will be a liquid or a
solid. If energy is increasing, the result is liquid water. If the energy is
decreasing, the result is ice.
Notice on the phase
change diagram below that, at most places, both temperature and heat energy are
increasing. But at the melting point and boiling point, only heat energy is
increasing. It is the increasing kinetic energy among particles that initiates
the phase change.
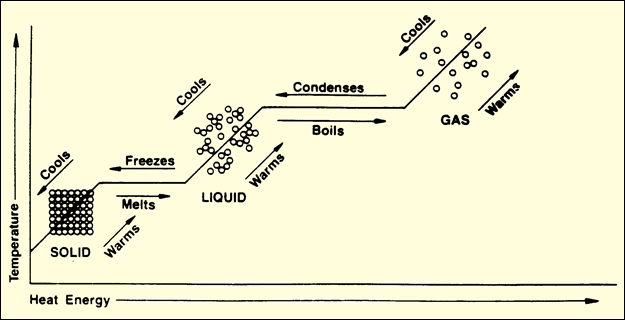
The melting point
is the point at which a solid will melt and turn into a liquid. The boiling
point
is the point at which a liquid will boil and turn into a gas. As a gas loses
heat energy and condenses, it turns into a liquid. As a liquid loses heat
energy and freezes, it turns into a solid.
Due to varying
composition, each substance has its own unique melting point and boiling point.
Therefore, the melting point and boiling point can be considered characteristic
properties of matter. They are physical properties because melting, freezing,
boiling, and condensing are all physical changes of matter.
When a solid gains enough
heat energy at once to turn straight into a gas, this is known as sublimation.
A good example of this is when snow on the ground turns to fog or water vapor
without melting first. Another example is dry ice. It goes directly from a
solid to a gaseous state in room temperature conditions.

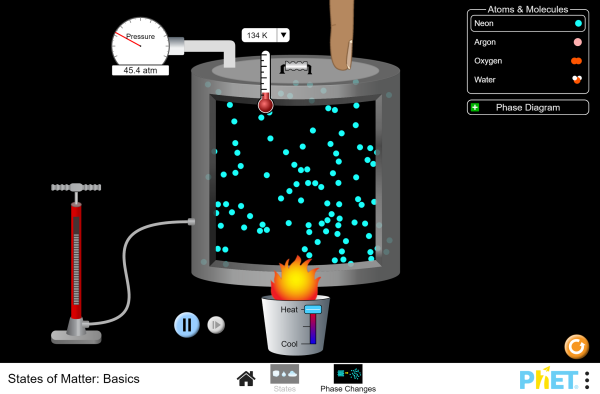
Now, take some time to
explore the following PhET simulation on states of matter and phase changes.
Answer the following
questions as you continue to explore the PhET simulation.
STUDENTS STATES OF MATTER BASICS DOCUMENT
Attach your completed
States of Matter Basics document to question #13 in the assessment portion of
the unit.
View the following video clip for more information on phase changes:
Quizlet Vocabulary