![j0287065[1]](SCIPSU08Chemical_Compounds_image003.png)
CHEMICAL
EQUATIONS and
WRITING
FORMULAS FOR COMPOUNDS
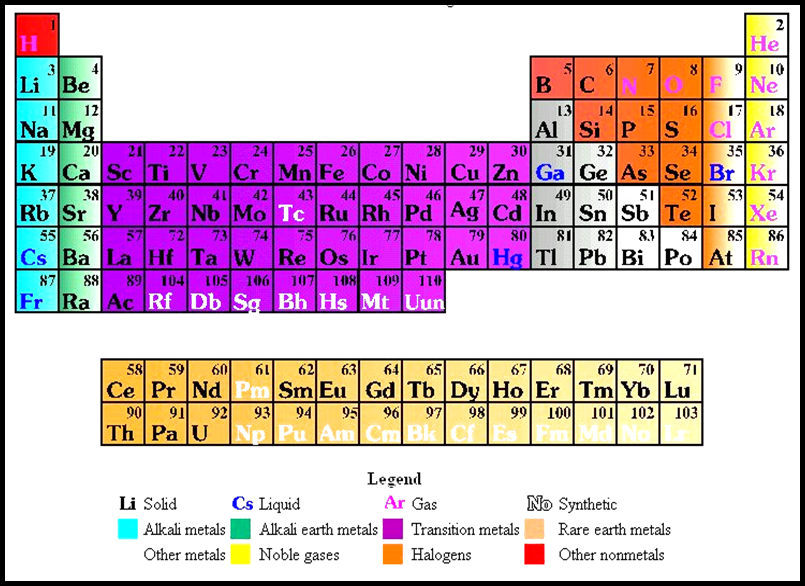
Once you are able to identify compounds and write formulas for compounds, the next step is the writing of a chemical equation. The function of a chemical equation is to show changes that occur during a chemical reaction. There are two ways to write a chemical equation. The first is a word equation that simply uses the words for the elements and compounds involved. An example would be Hydrogen plus Oxygen yields Water. The second type of chemical equation uses the symbols of those elements and compounds involved rather than the words.
|
An example of a
chemical equation would be:
|
The substances to the left of the arrow are called the reactants. Reactants are the elements and compounds that must interact in order for the reaction to take place. The elements and compounds to the right of the arrow are called the products. Products are those things produced in a chemical reaction. The arrow separates the reactants and products and is read as (à) yields. So the previous example read Hydrogen plus Oxygen yields Water. A chemical equation is much like a recipe. When baking, you need ingredients and, the ingredients in this formula would be the reactants. What you bake would be the products. Chocolate chips plus cookie dough make chocolate chip cookies.
In our previous example, you noticed that there is a 2 after the hydrogen and oxygen. This number is a subscript and is always written lower than the symbol. There are seven elements that are never found as one atom alone in nature. The seven diatomic elements are Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, and Iodine. They are described as being diatomic and always occur in pairs. This rule only applies when the element is by itself. If the element is part of a compound, then use the charges and criss-cross them. Sodium Chloride is NaCl. Notice, there is no 2 after the Cl. Na is +1 and Cl is -1 so we only need one of each.
|
|
In a chemical reaction, the atoms of substances are rearranged to produce new substances with different chemical and physical properties The substances you start out with are called reactants. The new substances that are formed are called the products.
A chemical equation uses formulas and
symbols to show what happens during a chemical reaction. The general form of a
chemical equation is: reactants→products
The coefficient is the large number that is in front of the atomic symbol. The subscript is the small number down to the left of the atomic symbol. The subscript can not be changed
The first step in balancing an equation is writing the formulas for all of the elements and compounds correctly. After the formulas have been initially written, the next step is to see if there is the same number of elements on both sides of the arrow. If the number of elements is not the same, then additional numbers, coefficients, must be added to make the number of elements the same. The number used to balance the equation is called a coefficient. These numbers are placed in front of the element or compound that is to be increased.
|
We will continue
with our first example: H2 + O2
à H2O |
Notice that there are two hydrogen’s on the left side and two hydrogen’s on the right side, making the hydrogen’s balanced. Now let’s look at the oxygen. There are two oxygen’s on the left and only one oxygen on the right side. Remember, you can not change the subscript; you must only use coefficients to balance. Here is what must be done.
|
H2 + O2
à 2H2O |
Notice a two was placed in front of the H2O. The two goes with the entire compound. Now there are four hydrogen’s. The two in front multiplied by the subscript two gives us four. The two in front of the H2O multiplied by the one Oxygen gives us 2 Oxygen’s. We now have two oxygen’s on each side. There is a problem however. By placing the two in front of the H2O, we have now messed up the Hydrogen. There are two on the left and four on the right. This can be fixed with another coefficient. If a two is placed in front of the H2 on the left, there will then be four Hydrogen’s. The final answer will look like:
|
2H2 + O2 à 2H2O The
number of elements on each side is balanced. |
|
Let’s try another example. Fe + Cl2 →
FeCl3 The
only places you can place a subscript is at the beginning of the equation,
after plus signs, or after the arrow.
In the above equation there are 3 places a subscript could be placed. Now
we count atoms on each side of the equation. Fe = 1 Fe = 1 Cl = 2 Cl
= 3 We
need to get the same number of chlorines on both sides of the equation. 2
and 3 both go into 6 so we need to get 6 chlorines on each side. Placing
a 3 in front of Cl2 will give 6 chlorines on the reactant
side. 3 x 2 = 6 Fe + 3
Cl2 →
FeCl3 Atom
count: Fe = 1 Fe = 1 Cl = 6
Cl = 3 Placing
a 2 on the reactant side will give us 6 chlorines, but it will also change
the number of irons. Fe + 3
Cl2 →2 FeCl3 A
new atom count gives: Fe = 1 Fe = 2 Cl = 6 Cl = 6 If
we go back to the reactant side of the equation and increase the number of
iron atoms the equation will be balanced. 2 Fe
+ 3 Cl2 →
2 FeCl3 |
In some chemical reactions, molecules split apart into atoms. In other reactions, atoms join together to form molecules. In yet other reactions, atoms change places with other atoms to form new molecules. Atoms are neither created nor destroyed in chemical reactions.
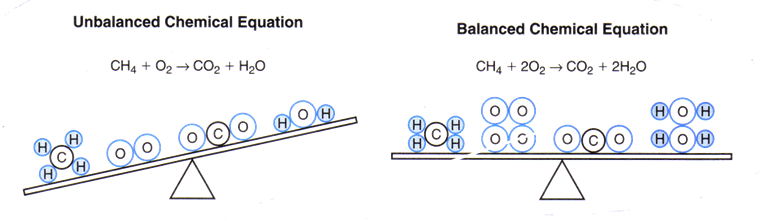
For example, consider what happens when methane burns in air. In this reaction, the reactants methane (CH4) and oxygen (O2) combine to form the products carbon dioxide (CO2) and water (H20). In the diagram above you see two possible chemical equations for this reaction. Why is the first equation unbalanced? In math you learn that the expressions on both sides of an equation must be equal. The same is true of chemical equations. In the first equation, there are four hydrogen atoms on the left side of the equation and only two on the right. There are two atoms of oxygen on the left side and three on the right. However, a chemical reaction must show that no atoms are created or destroyed in a chemical reaction.
Remember, the number of atoms of each element on each side of the equation must match. We do this by changing the number of units of a compound. We add an oxygen molecule (O2) to the left side. Then for the quantities to come out even there must be two water molecules (H20) on the right side.
|
Zn + HCl →
ZnCl2 + H2
If
you balanced it by placing a 2 in front of the HCl,
you are correct. |
How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!