![j0413592[1]](SCI170U26Global_Effect_image003.png)
GLOBAL
WARMING AND THE GREENHOUSE EFFECT
Unit Overview
The Earth’s climate has changed
dramatically in the past as great ice ages came and went. Those changes,
however, occurred over hundreds or thousands of years. Scientists are not sure
how quickly the Earth will continue to warm or how severe the effects will be.
In this unit, you will learn what causes global warming, the effects of
enhanced global warming, and some ways we can contribute to the slowing of
global warming.
The
Greenhouse Effect
The greenhouse effect is a natural process
by which some of the radiant heat from the Sun is captured in the lower
atmosphere of the Earth, thus maintaining the temperature of the Earth's surface.
Gases that help capture the heat, called “greenhouse gases,” include water
vapor, carbon dioxide, methane, nitrous oxide, and a variety of manufactured
chemicals. Some are emitted from natural sources while others result from human
activities.
The greenhouse effect is the
rise in temperature that the Earth experiences because certain gases in the
atmosphere (water vapor, carbon dioxide, nitrous oxide, and methane, for
example) trap energy (heat) from the sun. Without these gases, heat would escape
back into space and Earth’s average temperature would be about 60ºF colder. Because of
how they warm our world, these gases are referred to as greenhouse gases.
 Have you ever seen a greenhouse? Most greenhouses look like a small glass
house. Greenhouses are used to grow plants, especially in the winter, and work
by trapping heat from the sun. The glass panels of the greenhouse let in light,
but keep heat from escaping. This causes the greenhouse to heat up, much like
the inside of a car parked in sunlight, and keeps the plants warm enough to
live in the winter.
Have you ever seen a greenhouse? Most greenhouses look like a small glass
house. Greenhouses are used to grow plants, especially in the winter, and work
by trapping heat from the sun. The glass panels of the greenhouse let in light,
but keep heat from escaping. This causes the greenhouse to heat up, much like
the inside of a car parked in sunlight, and keeps the plants warm enough to
live in the winter.
The Earth’s atmosphere is all
around us. It is the air that we breathe and the air that plants utilize to
grow. Greenhouse gases in the atmosphere behave much like the glass panes in a
greenhouse. Sunlight enters the Earth's atmosphere, passing through the blanket
of greenhouse gases. As it reaches the Earth's surface, land, water, and
biosphere absorb the sunlight’s energy. Once absorbed, this energy is sent back
into our atmosphere. Some of the energy passes back into space, but much of it
remains trapped in our atmosphere by the greenhouse gases, causing our world to
heat up.
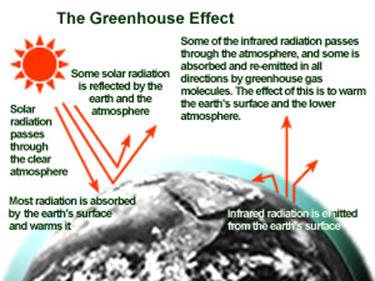
The greenhouse effect is
important. Without it, the Earth would not be warm enough for humans to live.
On the other hand, if the greenhouse effect were to become stronger, it could
make the Earth warmer than usual. Even a little extra warming may cause
problems for humans, plants, and animals.
![]() Global
Warming: The Greenhouse Effect (01:49)
Global
Warming: The Greenhouse Effect (01:49)
Introduction
In the last unit, we learned
the Goldilocks Principle can be summed up neatly as "Venus is too hot,
Mars is too cold, and Earth is just right." The fact that Earth has an average
surface temperature, comfortably between the boiling point and freezing point
of water and thus, suitable to sustain our sort of life, cannot be explained by
simply suggesting that our planet orbits at just the right distance from the
sun to absorb just the right amount of solar radiation. Our moderate
temperatures are also the result of having just the right kind of atmosphere. A
Venus-type atmosphere would produce unbearably hot conditions on our planet; a
Mars atmosphere would leave us shivering in a Martian-type deep freeze.

Instead, parts of our
atmosphere act as an insulating blanket of just the right thickness, trapping
sufficient solar energy to keep the global average temperature in a pleasant
range. The Martian atmospheric blanket is too thin, and the Venusian
atmospheric blanket is way too thick! The 'blanket' here is a collection of
atmospheric gases called 'greenhouse gases' based on the idea that the gases
also 'trap' heat like the glass walls of a greenhouse do. The ability of
certain trace gases to be relatively transparent to incoming visible light from
the sun, yet opaque to the energy radiated from the earth is one of the best
understood processes in the atmospheric sciences. This phenomenon, the
greenhouse effect, is what makes the earth habitable for life.
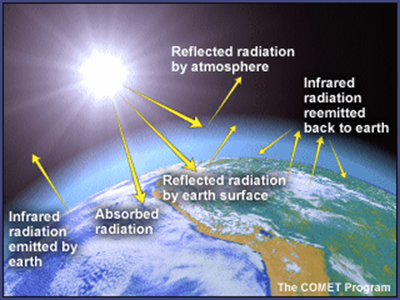
These gases, mainly water
vapor (![]() ), carbon dioxide (
), carbon dioxide (![]() ),
methane (
),
methane (![]() ),
and nitrous oxide (
),
and nitrous oxide (![]() ),
all act as effective global insulators. To understand why, it's important to
understand a few basic facts about solar radiation and the structure of
atmospheric gases.
),
all act as effective global insulators. To understand why, it's important to
understand a few basic facts about solar radiation and the structure of
atmospheric gases.
Solar Radiation
The sun radiates vast quantities
of energy into space, across a wide spectrum of wavelengths.
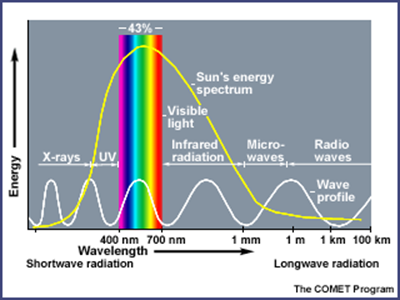
Most of the radiant energy
from the sun is concentrated in the visible and near-visible parts of the
spectrum. The narrow band of visible light, between 400 and 700 nm, represents
43% of the total radiant energy emitted. Wavelengths shorter than the visible
account for 7 to 8% of the total, but are extremely important because of their
high energy per photon. The shorter the wavelength of light, the more energy it
contains. Thus, ultraviolet light is very energetic (capable of breaking apart
stable biological molecules and causing sunburn and skin cancers). The
remaining 49 - 50% of the radiant energy is spread over the wavelengths longer
than those of visible light. These lie in the near infrared range from 700 to
1000 nm; the thermal infrared, between 5 and 20 microns; and the far infrared
regions. Various components of earth's atmosphere absorb ultraviolet and
infrared solar radiation before it penetrates to the surface, but the
atmosphere is quite transparent to visible light.
![]() Ultra-Violet
Rays: A Hidden Danger (01:45)
Ultra-Violet
Rays: A Hidden Danger (01:45)
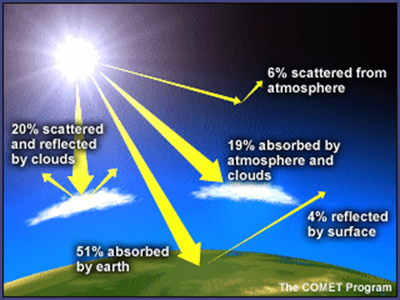
Absorbed by land, oceans, and
vegetation at the surface, the visible light is transformed into heat and
re-radiates in the form of invisible infrared radiation. If that was all there was
to the story, then during the day earth would heat up, but at night, all the
accumulated energy would radiate back into space and the planet's surface
temperature would fall far below zero very rapidly. The reason this doesn't
happen is that earth's atmosphere contains molecules that absorb the heat and
re-radiate the heat in all directions. This reduces the heat radiated out to
space. Called 'greenhouse gases' because they serve to hold heat in like the
glass walls of a greenhouse, these molecules are responsible for the fact that
the earth enjoys temperatures suitable for our active and complex biosphere.
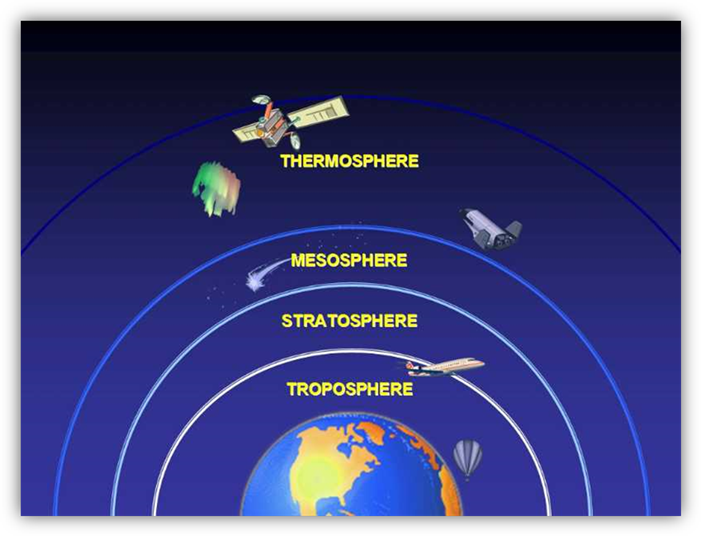
These greenhouse gases are
held in the atmosphere. The atmosphere
contains four major layers; these are the troposphere, the stratosphere, the
mesosphere, and the thermosphere. These layers are created because of the
different molecules, temperatures and air pressures found in the
atmosphere. You also need to remember
that the closer you get to space, the less gravity affects you.
Let’s start with the lowest
and densest layer of the atmosphere, the troposphere. This layer starts at Earth’s surface and
extends about 6 miles upward. Due to the
stored radiant energy, the troposphere is warmest near the surface of the
Earth. As we travel up through this
layer, temperature begins to drop to about -51 Celsius. As the altitude increases, the air pressure
decreases. All Earth’s weather happens
in the troposphere. This layer of the
atmosphere is about 78% nitrogen, 21% oxygen, and 1% other trace gases.
The stratosphere comes next
and it is the next densest layer. This
layer starts at 7 miles from the Earth and extends to 31 miles above Earth’s
surface. The stratosphere increases in
temperature from a low -51 degrees Celsius to -3 degrees Celsius. A major component of this layer is the Ozone
Layer. This is a belt of O3 molecules,
three oxygens connected together. The
Ozone layer is responsible for absorbing extreme amounts of ultraviolet
radiation. The space below the Ozone
Layer is cooler than that above because the Ozone keeps the heat from the sun’s
energy is the higher levels of the stratosphere. You will learn more about the Ozone later in
this unit.
The third level is the
mesosphere; it ranges from 31 to 53 miles above Earth’s surface. This layer starts off -3 degrees Celsius and
continues to get colder to -90 degrees Celsius.
Little is known about the mesosphere, because it is too high for air
craft and too low for space craft. But
we do know that in this layer meteorites heading towards Earth burn up,
creating shooting stars.
The last layer is the
thermosphere. The highest part of this
layer reaches 621 miles high. The
temperature increases from -90 degrees Celsius to 1000 degrees. There are few molecules this layer, but the
molecules that are there absorb great amounts of energy. Temperate measures the energy of the
molecules, not the amount of heat. So
even though the temperature reads that it is extremely hot, it’s not as hot as
you might think. This is also where the
Aurora Borealis or Northern Lights and the Aurora Australis or Southern Lights
occur. These lights in the sky happen
when electrons collide in this layer. The thermosphere is the home to the
International Space Station. This is a space craft that is housing humans and
used as a science lab.
To explore more about the
International Space Station click the link below.
http://www.nasa.gov/audience/forstudents/k-4/stories/nasa-knows/what-is-the-iss-k4.html

Greenhouse
Gases
Carbon dioxide (![]() )
is one of the greenhouse gases. It consists of one carbon atom with an oxygen
atom bonded to each side. When its atoms are bonded tightly together, the
carbon dioxide molecule can absorb infrared radiation and the molecule starts
to vibrate. Eventually, the vibrating molecule will emit the radiation again,
and it will likely be absorbed by yet another greenhouse gas molecule. This
absorption-emission-absorption cycle serves to keep the heat near the surface,
effectively insulating the surface from the cold of space.
)
is one of the greenhouse gases. It consists of one carbon atom with an oxygen
atom bonded to each side. When its atoms are bonded tightly together, the
carbon dioxide molecule can absorb infrared radiation and the molecule starts
to vibrate. Eventually, the vibrating molecule will emit the radiation again,
and it will likely be absorbed by yet another greenhouse gas molecule. This
absorption-emission-absorption cycle serves to keep the heat near the surface,
effectively insulating the surface from the cold of space.
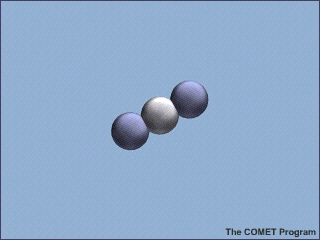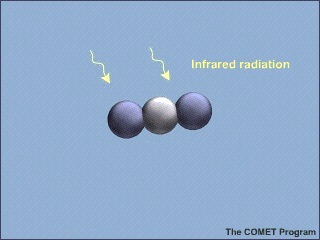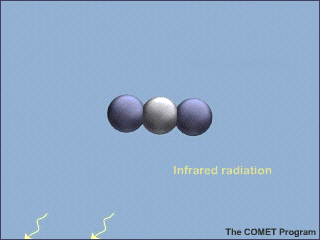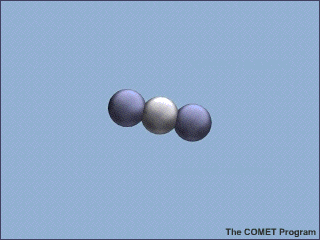
Carbon dioxide, water vapor (![]() ),
methane (
),
methane (![]() ),
nitrous oxide (
),
nitrous oxide (![]() ),
and a few other gases are greenhouse gases. They all are molecules composed of
more than two component atoms, bound loosely enough together to be able to
vibrate with the absorption of heat. The major components of the atmosphere (
),
and a few other gases are greenhouse gases. They all are molecules composed of
more than two component atoms, bound loosely enough together to be able to
vibrate with the absorption of heat. The major components of the atmosphere (![]() and
and ![]() )
are two-atom molecules too tightly bound together to vibrate and thus they do
not absorb heat and contribute to the greenhouse effect.
)
are two-atom molecules too tightly bound together to vibrate and thus they do
not absorb heat and contribute to the greenhouse effect.
![]() How the Energy of
Large Cities Affects Weather and Climate (03:19)
How the Energy of
Large Cities Affects Weather and Climate (03:19)
Greenhouse Effect
Atmospheric scientists first
used the term 'greenhouse effect' in the early 1800s. At that time, it was used
to describe the naturally occurring functions of trace gases in the atmosphere
and did not have any negative connotations. It was not until the mid-1950s that
the term greenhouse effect was coupled with concern over climate change. In recent
decades, we often hear about the greenhouse effect in somewhat negative terms.
The negative concerns are related to the possible impacts of an enhanced
greenhouse effect. It is important to
remember that without the greenhouse effect, life on earth as we know it would
not be possible.
The greenhouse effect is a
natural process by which some of the radiant heat from the Sun is captured in
the lower atmosphere of the Earth, thus maintaining the temperature of the
Earth's surface. The gases that help capture the heat, called “greenhouse
gases,” include water vapor, carbon dioxide, methane, nitrous oxide, and a
variety of manufactured chemicals. Some are emitted from natural sources;
others are anthropogenic, resulting from human activities.
Over the past several
decades, rising concentrations of greenhouse gases have been detected in the
Earth's atmosphere. Although there is not universal agreement within the
scientific community on the impacts of increasing concentrations of greenhouse
gases, it has been theorized that they may lead to an increase in the average
temperature of the Earth's surface. To date, it has been difficult to note such
an increase conclusively because of the differences in temperature around the
Earth and throughout the year, and because of the difficulty of distinguishing
permanent temperature changes from the normal fluctuations of the Earth's
climate. In addition, there is not universal agreement among scientists and
climatologists on the potential impacts of an increase in the average
temperature of the Earth, although it has been hypothesized that it could lead
to a variety of changes in the global climate, sea level, agricultural
patterns, and ecosystems that could be, on average, detrimental.
While the earth's temperature
is dependent upon the greenhouse-like action of the atmosphere, the amount of
heating and cooling are strongly influenced by several factors just as
greenhouses are affected by various factors.
In the atmospheric greenhouse
effect, the type of surface that sunlight first encounters is the most
important factor. Forests, grasslands, ocean surfaces, ice caps, deserts, and
cities all absorb, reflect, and radiate radiation differently. Sunlight falling
on a white glacier surface strongly reflects back into space, resulting in
minimal heating of the surface and lower atmosphere. Sunlight falling on a dark
desert soil is strongly absorbed, on the other hand, and contributes to
significant heating of the surface and lower atmosphere. Cloud cover also affects
greenhouse warming by both reducing the amount of solar radiation reaching the
earth's surface and by reducing the amount of radiation energy emitted into
space.
Scientists use the term albedo
to define the percentage of solar energy reflected back by a surface.
Understanding local, regional, and global albedo effects is critical to
predicting global climate change. Some factors that influence the earth's
albedo are summarized below.
·
Clouds:
On a hot, sunny day, we
usually welcome a big fluffy cumulus cloud passing overhead because we feel
cooler immediately. That's because the top of the cloud reflects sunlight back
into space before it ever reaches earth. Depending on their altitude and
optical properties, clouds either cool or warm the earth. Large, thick,
relatively low-altitude clouds, such as cumulus and cumulonimbus, reflect
incoming solar radiation and thereby reduce warming of the surface. The
whitewash on plant greenhouses has the same effect on a smaller scale.
High-altitude, thinner clouds, such as cirrus clouds, absorb long wave
radiation reflected from the earth's surface, causing increased warming.
|
|
|
|
|
Cirrus |
Cumulus |
Nimbus |
·
Surface
albedo: Just as some
clouds reflect solar energy into space, so do light-colored land surfaces. This
surface albedo effect strongly influences the absorption of sunlight. Snow and
ice cover are highly reflective, as are light-colored deserts. Large expanses
of reflective surfaces can significantly reduce solar warming. Dark-colored
land surfaces, in contrast, are strongly absorptive and contribute to warming.
If global temperatures increase, snow and ice cover may shrink. The exposed
darker surfaces underneath may absorb more solar radiation, causing further
warming. The magnitude of the effect is currently a matter of serious
scientific study and debate.
·
Oceans: From space, oceans look much different
than adjacent land areas - they often appear darker, suggesting that they
should be absorbing far more sunlight. But unlike dry land, water absorbs
energy in a dynamic fashion. Some of the solar energy contacting the surface
may be carried away by currents, some may go into producing water vapor, and some
may penetrate the surface and be mixed meters deep into the water column. These
factors combine to make the influence of the ocean surface an extremely complex
and difficult phenomenon to predict.
Water also has the capacity to store heat and transport large amounts of heat
energy. In addition, oceans are an important sink (storage site) for
atmospheric carbon dioxide, and their ability to absorb ![]() is strongly related to ocean temperature.
Because of their enormous size and depth, oceans are extremely important in
determining global climate and the future rate of global temperature change.
is strongly related to ocean temperature.
Because of their enormous size and depth, oceans are extremely important in
determining global climate and the future rate of global temperature change.
·
Forested
areas: Like the oceans,
the interaction of forests and sunlight is complex. The amount of solar
radiation absorbed by forest vegetation depends upon the type and color of
vegetation, the time of year, and how well watered and healthy the plants are.
In general, plants provide a dark surface, so you might expect high solar
absorption. A significant fraction of the solar radiation is captured by the
plants and used to make food through photosynthesis (and thus it doesn't
re-radiate as heat); some of the energy is dissipated as water evaporates from
plant leaves; and some is absorbed and distributed deep within the forest
canopy. These complexities make a simple definition of forest influences
impossible. To a lesser extent, the same complexities apply to any relatively
continuous-cover ecosystem (for example, grasslands and farmlands).
Global Warming
Global warming is the
gradual increase of the temperature of the Earth's lower atmosphere as a result
of the increase in greenhouse gases since the Industrial Revolution.
The temperature of the
atmosphere near the earth's surface is warmed through a natural process called
the greenhouse effect. Visible, shortwave light comes from the sun to the earth,
passing unimpeded through a blanket of thermal, or greenhouse, gases composed
largely of water vapor, carbon dioxide, methane, nitrous oxide, and ozone. Infrared
radiation reflects off the planet's
surface toward space but does not easily pass through the thermal blanket. Some
of it is trapped and reflected downward, keeping the planet at an average
temperature suitable to life, about 60°F (16°C).
Growth in industry,
agriculture, and transportation since the Industrial Revolution has produced
additional quantities of natural greenhouse gases plus chlorofluorocarbons and
other gases, augmenting the thermal blanket. It is generally accepted that this
increase in the quantity of greenhouse gases is trapping more heat and
increasing global temperatures, making a process that has been beneficial to
life potentially disruptive and harmful. During the past century, the
atmospheric temperature has risen 1.1°F (0.6°C), and sea level has risen
several inches. Some projected, longer-term results of global warming include
melting of polar ice, with a resulting rise in sea level and coastal flooding;
disruption of drinking water supplies dependent on snow melts; profound changes
in agriculture due to climate change; extinction of species as ecological
niches disappear; more frequent tropical storms; and an increased incidence of
tropical diseases.
Among factors that may be
contributing to global warming are the burning of coal and petroleum products
(sources of carbon dioxide, methane, nitrous oxide, ozone); deforestation,
which increases the amount of carbon dioxide in the atmosphere; methane gas
released in animal waste; and increased cattle production, which contributes to
deforestation, methane production, and use of fossil fuels.
Much of the debate
surrounding global warming has centered on the accuracy of scientific
predictions concerning future warming. To predict global climatic trends,
climatologists accumulate large historical databases and use them to create
computerized models that simulate the earth's climate. The validity of these
models has been a subject of controversy. Skeptics say that the climate is too
complicated to be accurately modeled, and that there are too many unknowns.
Some also question whether the observed climate changes might simply represent
normal fluctuations in global temperature. Nonetheless, for some time there has
been general agreement that at least part of the observed warming is the result
of human activity, and that the problem needs to be addressed. In 1992, at the
United Nations Conference on
Environment and Development, over
150 nations signed a binding declaration on the need to reduce global warming.
In 1994, however, a UN
scientific advisory panel, the Intergovernmental Panel on Climate Change,
concluded that reductions beyond those envisioned by the treaty would be needed
to avoid global warming. The following year, the advisory panel forecast a rise
in global temperature of from 1.44 to 6.3°F (0.8–3.5°C) by 2100 if no action is
taken to cut down on the production of greenhouse gases, and a rise of from 1
to 3.6°F (0.5–2°C) even if action is taken (because of already released gases
that will persist in the atmosphere).
A UN Conference on Climate
Change, held in Kyoto, Japan, in 1997 resulted in an international agreement to
fight global warming, which called for reductions in emissions of greenhouse
gases by industrialized nations. Not all industrial countries, however,
immediately signed or ratified the accord. In 2001 the G. W. Bush
administration announced it would abandon the Kyoto Protocol; because the
United States produces about one quarter of the world's greenhouse gases, this
was regarded as a severe blow to the effort to slow global warming. Despite the
American move, most other nations agreed later in the year (in Bonn, Germany,
and in Marrakech, Morocco) on the details necessary to convert the agreement
into a binding international treaty.
Improved automobile mileage,
reforestation projects, energy efficiency in construction, and national support
for mass transit are among relatively simpler adjustments that could
significantly lower U.S. production of greenhouse gases. More aggressive
adjustments include a gradual worldwide shift away from the use of fossil
fuels, the elimination of chlorofluorocarbons, and the slowing of deforestation
by restructuring the economies of developing nations. In 2002 the Bush
administration proposed several voluntary measures for slowing the increase in,
instead of reducing, emissions of greenhouses gases.
The Ozone Layer
The Earth's atmosphere is
divided into several layers. The lowest region, the troposphere, extends from
the Earth's surface up to about 10 kilometers (km) in altitude. The next layer,
the stratosphere, continues from 10 km to about 50 km. Most atmospheric ozone
is concentrated in a layer in the stratosphere, about 15–30 kilometers above
the Earth's surface.
Ozone is a molecule
containing three oxygen atoms. It is blue in color and has a strong odor.
Normal oxygen, which we breathe, has two oxygen atoms and is colorless and
odorless. Ozone is much less common than normal oxygen. Out of each 10 million
air molecules, about 2 million are normal oxygen, but only 3 are ozone.
However, even the small
amount of ozone plays a key role in the atmosphere. The ozone layer absorbs a
portion of the radiation from the sun, preventing it from reaching the planet's
surface. Most importantly, it absorbs the portion of ultraviolet light called
UVB. UVB has been linked to many harmful effects, including various types of
skin cancer, cataracts, and harm to some crops, certain materials, and some
forms of marine life.
At any given time, ozone
molecules are constantly formed and destroyed in the stratosphere. The total
amount, however, remains relatively stable. While ozone concentrations vary
naturally with sunspots, the seasons, and latitude, these processes are well
understood and predictable. Each natural reduction in ozone levels has been
followed by a recovery. Recently, however, convincing scientific evidence has
shown that the ozone shield is being depleted well beyond changes due to
natural processes.
![]() Ozone:
Harmful and Helpful (03:56)
Ozone:
Harmful and Helpful (03:56)
Ozone Depletion
For over 50 years,
chlorofluorocarbons, or CFCs, were thought of as miracle substances. They are
stable, nonflammable, low in toxicity, and inexpensive to produce. Over time,
CFCs found uses as refrigerants, solvents, foam blowing agents, and in other
smaller applications. Other chlorine-containing compounds include methyl
chloroform, a solvent, and carbon tetrachloride, an industrial chemical.
Halons, extremely effective fire extinguishing agents, and methyl bromide, an
effective produce and soil fumigant, contain bromine. All of these compounds
have atmospheric lifetimes long enough to allow them to be transported by winds
into the stratosphere. Because they release chlorine or bromine when they break
down, they damage the protective ozone layer.
In the early 1970s,
researchers began to investigate the effects of various chemicals on the ozone
layer, particularly CFCs, which contain chlorine. They also examined the
potential impacts of other chlorine sources. Chlorine from swimming pools,
industrial plants, sea salt, and volcanoes does not reach the stratosphere.
Chlorine compounds from these sources readily combine with water and repeated
measurements show that they rain out of the troposphere very quickly. In
contrast, CFCs are very stable and do not dissolve in rain. Thus, there are no
natural processes that remove the CFCs from the lower atmosphere. Over time,
winds drive the CFCs into the stratosphere.
The CFCs are so stable that
only exposure to strong UV radiation breaks them down. When that happens, the
CFC molecule releases atomic chlorine. One chlorine atom can destroy over
100,000 ozone molecules. The net effect is to destroy ozone faster than it is
naturally created.
Why is the ozone
layer important?
The ozone layer is a
concentration of ozone molecules in the stratosphere. About 90% of the planet's
ozone is in the ozone layer. The layer of the Earth's atmosphere that surrounds
us is called the troposphere. The stratosphere, the next higher layer, extends
about 10–50 kilometers above the Earth's surface. Stratospheric ozone is a
naturally occurring gas that filters the sun's ultraviolet (UV) radiation. A
diminished ozone layer allows more radiation to reach the Earth's surface. For
people, overexposure to UV rays can lead to skin cancer, cataracts, and
weakened immune systems. Increased UV can also lead to reduced crop disruptions
in the marine food chain, and other harmful effects.
How does ozone
depletion occur?
It is caused by the release
of chlorofluorocarbons (CFCs) and other ozone-depleting substances (ODS), which
were used widely as refrigerants, insulating foams, and solvents. The discussion
below focuses on CFCs, but is relevant to all ODS. Although CFCs are heavier
than air, they are eventually carried into the stratosphere in a process that
can take as long as 2 to 5 years.
When CFCs reach the
stratosphere, the ultraviolet radiation from the sun causes them to break apart
and release chlorine atoms, which react with ozone, starting chemical cycles of
ozone destruction that deplete the ozone layer. One chlorine atom can break
apart more than 100,000 ozone molecules.
Other chemicals that damage
the ozone layer include methyl bromide (used as a pesticide) and halons (used
in fire extinguishers). As methyl bromide and halons are broken apart, they
release bromine atoms, which are 40 times destructive to ozone molecules than
chlorine atoms.
How do we know that
natural sources are not responsible for ozone depletion?
While it is true that
volcanoes and oceans release large amounts of chlorine, the chlorine from these
sources is easily dissolved in water and washes out of the atmosphere in rain.
In contrast, CFCs are not broken down in the lower atmosphere and do not
dissolve in water. The chlorine in these human-made molecules does reach the
stratosphere. Measurements show that the increase in stratospheric chlorine
since 1985 matches the amount released from CFCs and other ozone-depleting
substances produced and released by human activities.
What is being done
about ozone depletion?
In 1978, the use of CFC
propellants in spray cans was banned in the U.S. In the 1980s, the Antarctic “ozone
hole” appeared and an international science assessment more strongly linked the
release of CFCs and ozone depletion. It became evident that a stronger
worldwide response was needed. In 1987, the Montreal Protocol was signed and
the signatory nations committed themselves to a reduction in the use of CFCs
and other ozone-depleting substances.
Since that time, the treaty
has been amended to ban CFC production after 1995 in the developed countries,
and later in developing. Today, over 160 countries have signed the treaty.
Beginning January 1, 1996, only recycled and stockpiled CFCs will be available
for use in developed countries like the US. This production phase-out is
possible because of efforts to ensure that there will be substitute chemicals
and technologies for all CFC uses.
Will the ozone layer
recover? Can we make more ozone to fill in the hole?
The answers, in order, are:
yes and no. We can't make enough ozone to replace what's been destroyed, but
provided that we stop producing ozone-depleting substances, natural ozone
production reactions should return the ozone layer to normal levels by about
2050. It is very important that the world comply with the Montreal Protocol;
delays in ending production could result in additional damage and prolong the
ozone layer's recovery.
How do we impact the
ozone layer and global warming?
Earth has warmed by about 1ºF
over the past 100 years. But why? And how? Well, scientists are not exactly
sure. The Earth could be getting warmer on its own, but many of the world’s
leading climate scientists think that things people do are helping make the
Earth warmer.
The Greenhouse Effect: Scientists are sure about the greenhouse effect. They
know that greenhouse gases make the Earth warmer by trapping energy in the atmosphere.
Climate Change:
Climate is the long-term average of a region's weather events lumped together.
For example, it's possible that a winter day in Buffalo, New York, could be sunny
and mild, but the average weather – the climate – tells us that Buffalo's
winters will mainly be cold and include snow and rain. Climate change
represents a change in these long-term weather patterns. They can become warmer
or colder. Annual amounts of rainfall or snowfall can increase or decrease.
Global Warming:
Global warming refers to an average increase in the Earth's temperature, which
in turn causes changes in climate. A warmer Earth may lead to changes in
rainfall patterns, a rise in sea level, and a wide range of impacts on plants,
wildlife, and humans. When scientists talk about the issue of climate change,
their concern is about global warming caused by human activities.
Can We Change the
Climate?
It may seem hard to believe
that people can actually change the Earth’s climate. But scientists think that
the things people do that send greenhouse gases into the air are  making our
planet warmer.
making our
planet warmer.
Once, all climate changes
occurred naturally. However, during the Industrial Revolution, we began altering our
climate and environment
through agricultural and industrial practices. The Industrial Revolution
was a time when people began using machines to make life easier. It started
more than 200 years ago and changed the way humans live. Before the Industrial
Revolution, human activity released very few gases into the atmosphere, but now
through population growth, fossil fuel burning, and deforestation, we are affecting
the mixture of gases in the atmosphere.
Since the Industrial
Revolution, the need for energy to run machines has steadily increased. Some
energy, like the energy you need to do your homework, comes from the food you
eat. But other energy, like the energy that makes cars run and much of the
energy used to light and heat our homes, comes from fuels like coal and oil –
fossil fuels. Burning these fuels releases greenhouse gases.
When Do You Send
Greenhouse Gases into the Air?
Whenever you ….
·
Watch TV
·
Play a Video Game
![]()
·
Listen to a
Stereo ![]()
·
Use the Air
Conditioner ![]()
·
Turn on a Light ![]()
·
Wash or Dry Clothes
![]()
·
Use a Hair Dryer ![]()
·
Use a Dish Washer
![]()
·
Ride in a Car
·
Microwave a Meal
![]() .... You are helping to send greenhouse gas into
the air.
.... You are helping to send greenhouse gas into
the air.
To perform many of these
functions, you need to use electricity. Electricity comes from power plants. Most
power plants use coal and oil to make electricity. Burning coal and oil
produces greenhouse gases.

Other things we do send greenhouse gases into the air
too;
![]() The trash that we send to landfills produces a
greenhouse gas called methane. Methane is also produced by the animals we raise
for dairy and meat products and when we take coal out of the ground. Whenever
we drive or ride in a car, we are adding greenhouse gases to the atmosphere.
And, when factories make the things that we buy and use everyday, they too are
sending greenhouse gases into the air.
The trash that we send to landfills produces a
greenhouse gas called methane. Methane is also produced by the animals we raise
for dairy and meat products and when we take coal out of the ground. Whenever
we drive or ride in a car, we are adding greenhouse gases to the atmosphere.
And, when factories make the things that we buy and use everyday, they too are
sending greenhouse gases into the air.
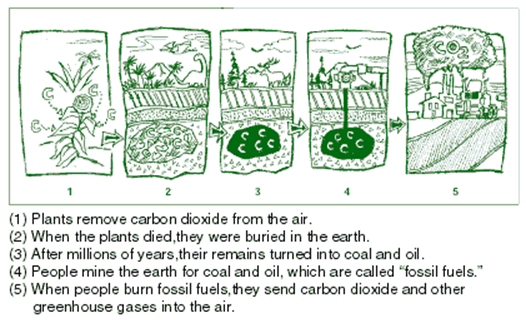
We Can Make a
Difference!
Global warming may be a big
problem, but there are many little things we can do to make a difference. If we
try, most of us can do our part to reduce the amount of greenhouse gases that we put into
the atmosphere. Many greenhouse gases come from things we do every day. As we
have learned, these greenhouse gases trap energy in the atmosphere and make the
Earth warmer.
Driving a car or using
electricity is not wrong. We just have to be smart about it. Some people use
less energy by carpooling. For example, four people can ride together in one
car instead of driving four cars to work. Here are some additional ways you can
help make the planet a better place!
Read
L![]() earning
about the environment
is very important. There are many good books that will help you
learn. To get started, ask a teacher or a librarian for some suggestions. You
also can look at the Links page to find other good web sites with information
about the environment and climate change.
earning
about the environment
is very important. There are many good books that will help you
learn. To get started, ask a teacher or a librarian for some suggestions. You
also can look at the Links page to find other good web sites with information
about the environment and climate change.
Save
Electricity
Using electricity, puts greenhouse gases into the air. By turning off lights,
the television, and the computer when you are through with them, you can help a
lot.
Bike, Bus, and
Walk
![]() Taking
the bus, riding a bike, or walking saves energy.
Taking
the bus, riding a bike, or walking saves energy.
Talk to Your
Family and Friends
![]() Talk
with your family and friends about global warming. Let them know what you've
learned.
Talk
with your family and friends about global warming. Let them know what you've
learned.
Plant Trees
Planting trees is fun and a great way to reduce greenhouse gases. Trees absorb
carbon dioxide, a greenhouse gas, from the air.
Recycle
![]() Recycle
cans, bottles, plastic bags, and newspapers. When you recycle, you send less trash
to the landfill and you help save natural resources, like trees, oil, and
elements such as aluminum.
Recycle
cans, bottles, plastic bags, and newspapers. When you recycle, you send less trash
to the landfill and you help save natural resources, like trees, oil, and
elements such as aluminum.
When You Buy,
Buy Cool Stuff
![]() There
are lots of ways we can improve the environment. One of the ways to reduce the
amount of greenhouse gases that we put into the air is to buy products that
don't use as much energy. By conserving energy, we help reduce global warming
and make the Earth a better place. Some products – like certain cars and
stereos – are made specially to save energy.
There
are lots of ways we can improve the environment. One of the ways to reduce the
amount of greenhouse gases that we put into the air is to buy products that
don't use as much energy. By conserving energy, we help reduce global warming
and make the Earth a better place. Some products – like certain cars and
stereos – are made specially to save energy.
Some Things to Think About
Did you know you can help
the environment if you buy recyclable products instead of non-recyclable ones?
Look for the recycle mark – three arrows that make a circle – on the package.
Recyclable products are usually made out of things that already have been used.
It usually takes less energy to make recycled products than to make new ones.
The less energy we use, the better.
Cars
![]() Cars
are an important part of life for most people. But cars also cause pollution
and release a lot of greenhouse gases into the air. Fortunately, there are some
cars that are better for the environment. These cars can travel longer on a
smaller amount of gasoline. They don't pollute as much, either. Using these
kinds of cars can help reduce the amount
Cars
are an important part of life for most people. But cars also cause pollution
and release a lot of greenhouse gases into the air. Fortunately, there are some
cars that are better for the environment. These cars can travel longer on a
smaller amount of gasoline. They don't pollute as much, either. Using these
kinds of cars can help reduce the amount  of
greenhouse gases in the air.
of
greenhouse gases in the air.
ENERGY
STAR
![]() Many things, like computers, TVs, stereos, and VCRs,
have special labels on them. The label says "Energy" and has a
picture of a star. Products with the ENERGY STAR® label are made to
save energy. Buying products with ENERGY STAR® labels will help
protect the environment.
Many things, like computers, TVs, stereos, and VCRs,
have special labels on them. The label says "Energy" and has a
picture of a star. Products with the ENERGY STAR® label are made to
save energy. Buying products with ENERGY STAR® labels will help
protect the environment.
Solar Energy
Imagine that it's a hot summer day. You put a scoop of ice
cream on the sidewalk, and it melts. Why? Well, you probably know that the sun
causes the ice cream to melt. But you may not know that the sun produces solar
energy. Solar energy is a fancy way of saying "energy that comes from the
sun." Solar energy can be used to heat homes, buildings, water, and to
make electricity. Today, more than 200,000 houses in the United States take
advantage of the sun's energy.
 Now answer questions
1 through 30.
Now answer questions
1 through 30.

Below are additional educational resources and activities for this unit.
Unit 26 Climate Change Activity


