ISOTOPES
Unit Overview
In this unit we will explore
isotopes, how they are used, and how they are written.
Isotopes
All matter is made of atoms. Until the late 1800ís
scientists believed that atoms were the smallest particles to exist. Atoms are
actually composed of subatomic particles called protons, neutrons and
electrons. The nucleus, the centrally located part of the atom containing over
99% of the mass, consists of protons and neutrons. The area surrounding the
nucleus is called the electron cloud. The electrons travel in three dimensional
geometrically shaped orbits at such a high rate of speed that they effectively
occupy the entire volume of the electron cloud.
The number of protons an atom has in its nucleus is
known as the ATOMIC NUMBER. This number is unique to each different kind of atom
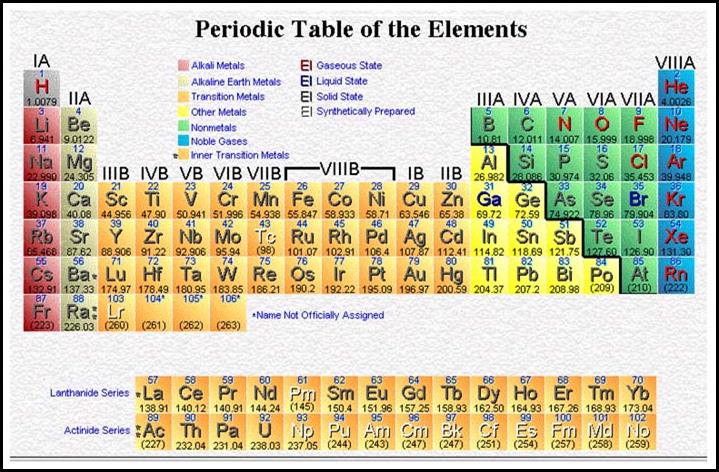
and is the whole number found for each element on the periodic table
(www.periodictable.com). You will want to download and print off a copy of the
table now if you donít have a copy.
Printable version of
the Periodic Table
You should remember that atoms are neutral, meaning;
they have no overall positive or negative charge. Therefore, the number of
protons always equals the number of electrons. For instance, calciumís atomic
number is 20. This means it has 20 protons and 20 electrons. Ironís atomic
number is 25. Therefore it has 25 protons and 25 electrons.
You will also see an atomic mass or weight printed in
each block for the elements. This will not be a whole number in most cases.
The atomic masses shown in parentheses are estimates, indicating that the
element is RADIOACTIVE and is
spontaneously emitting particles thus changing its mass.
All the atoms of a particular type of element do not necessarily
have the same mass. Even though the number of protons and electrons always
stays the same for a particular type of element, the number of neutrons may
differ. Atoms of the same element having the same number of protons and
electrons but different numbers of neutrons are known as ISOTOPES.
The atoms of elements like aluminum, fluorine, gold
and phosphorus exist in only one form. Tin, however, exists in ten different
forms, each differing only in mass due to the different numbers of neutrons.
Most elements exist only in two or three different
forms. There are three isotopes of hydrogen:
|
ISOTOPES |
PROTONS |
NEUTRONS |
ELECTRONS |
|
Protium: Hydrogen-1 |
1 |
0 |
1 |
|
Deuterium: Hydrogen-2 |
1 |
1 |
1 |
|
Tritium: Hydrogen-3 |
1 |
2 |
1 |
(Notice that the number of protons and electrons stays
the same, only the number of neutrons varies.)
There are three different isotope notations. The first
is the elementís name followed by a hyphen and itís MASS NUMBER (Ex.
carbon-14). The mass number is the sum of the protons plus neutrons in the
nucleus. The second notation simply uses the elementís atomic symbol followed
by its mass number (Ex. C-14). The third notation is known as the NUCLEAR NOTATION. It uses the symbol for the element with its mass
number written to the upper left and its atomic number written to the lower
left:
 ††††††††††††††††
††††††††††††††††  ††††††††††††††††
††††††††††††††††
Any of the notations may be used to determine the
number of protons, neutrons and electrons that the atoms of that isotope may
contain. The first two notations require the use of the periodic table to
obtain the elementís atomic number. This represents the number of protons as
well as the number of electrons. Example: zinc-65. The atomic number of zinc is
30 so it has 30 protons and 30 electrons. The atomic number is then subtracted from
the mass number to determine the number of neutrons.
|
|
PROTONS |
NEUTRONS |
ELECTRONS |
|
Zinc-65 |
30 |
65-30 = 35 |
30 |
|
Cl-35 |
17 |
35-17 = 18 |
17 |
|
P-31 |
15 |
31-15 = 1 |
15 |
![]() ††† What do you know about the Periodic Table?
††† What do you know about the Periodic Table?
The periodic table is not needed to determine particle counts when the nuclear notation is used. The lower number (atomic number) represents the protons as well as the electrons. The difference between the upper number (mass number) and lower number (atomic number) represents the number of neutrons.
Example:
|
|
PROTONS |
NEUTRONS |
ELECTRONS |
|
††† |
6 |
12-6=6 |
6 |
|
|
92 |
238-92=146 |
92 |
|
|
11 |
23-11=12 |
11 |
PRACTICE
ACTIVITY
The following periodic table is missing a few parts.
The cells are all present in the usual periodic table format. The group and
period numbers are labelled and a nice solid line separates the metals from the
metalloids and nonmetals. Test your knowledge of the elements by filling in the
missing elements.
Printable
Blank Periodic Table
 ††† Now answer
question 1 through 34.
††† Now answer
question 1 through 34.


