ENTROPY
Have you ever seen a ball or a rock roll uphill? Of course you haven’t—unless it was part of a magic trick to fool you. What happens when you hold a lit match to a dry piece of paper? It catches fire and burns up completely leaving only smoke and a pile of ashes. Why do these processes happen this way? These processes are SPONTANEOUS—they occur naturally by some internal cause.
Scientists have spent years researching what causes processes and reactions to occur the way they do. Their results show that processes in nature are driven in two ways: (1) toward a lower energy state and greater stability; and (2) toward greater disorder or randomness.
The ball rolls down the hill because at the bottom it has transformed all of its stored energy, its POTENTIAL ENERGY, into motion (KINETIC ENERGY). One reason the piece of paper becomes smoke and a pile of ashes is because the stored chemical energy is transformed into light and heat energy. This is an application of the FIRST LAW OF THERMODYNAMICS, also known as the LAW OF CONSERVATION OF ENERGY. It states that energy is neither created nor destroyed but is transformed from one form to another, and the total amount of energy in the universe is constant.
For additional information on Entropy click on the following link: What is Entropy
Examples of Potential and Kinetic
Energy
Potential Energy
 Potential
energy is energy that is in a stored form. It isn't being used at the moment,
but is waiting to do work. Think about a boulder sitting on top of a hill. Just
sitting there, the boulder isn't doing anything. But because it is sitting on
top of a hill, it has the potential to roll down and do some damage to a car or
building below. The energy is stored in that rock because of its size (mass)
and the distance it will travel once it starts rolling.
Potential
energy is energy that is in a stored form. It isn't being used at the moment,
but is waiting to do work. Think about a boulder sitting on top of a hill. Just
sitting there, the boulder isn't doing anything. But because it is sitting on
top of a hill, it has the potential to roll down and do some damage to a car or
building below. The energy is stored in that rock because of its size (mass)
and the distance it will travel once it starts rolling.

Kinetic
Energy
Kinetic energy is energy that is in motion. This energy is performing
work. Legs are pumping bicycle pedals. Coal is running generators. Lightning is
snapping trees.
For additional information on Potential and Kinetic Energy visit the following web site. http://www.geocities.com/yummyphysics/energy.html
You can click on the PDF File to see what the Home Page has to offer to find additional information on your topic. Potential and Kinetic Energy PDF File.
Heat is at the end of all useful transformations. Another reason why the piece of paper becomes
smoke and ashes is that these forms of the carbon, hydrogen and oxygen that
were once chemically combined and very orderly in the paper are now in a very
disordered state as smoke and ashes. The
amount of disorder or randomness of a system is known as ENTROPY. The SECOND LAW OF THERMODYNAMICS, also
known as the LAW OF ENTROPY, states
that the direction of spontaneous change in isolated systems is toward maximum
disorder. Heat always travels from an
area of higher temperature to an area of lower temperature. As the cooler area gains heat, the motion of
the cooler molecules becomes more disorderly the warmer they get and entropy
increases. If you’ve ever been scolded
for leaving the outside door of your home open because “you’re letting the cold
air in”, you are actually letting the warm air out! HEAT
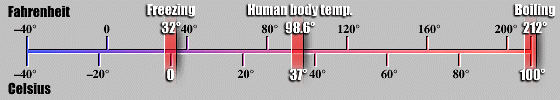
is a quantity of energy and is measured in CALORIES
or JOULES. TEMPERATURE
is a measure of the intensity of the heat, indicates the direction of the
flow, and is measured in degrees identified as Celsius, Fahrenheit, or Kelvin.
Looking around at our world we see many applications of the law of entropy. Here are a few examples:
- No matter how many times you shuffle a deck of any kind of cards (playing, UNO, etc.), they NEVER end up in an ordered sequence.
- When you put air into a balloon, it NEVER clumps together in one small portion of the interior but immediately spreads out to fill the interior of the balloon. Some of the energy of the disordered particles is also transferred to the balloon’s elastic material. As it expands, it gets warmer.
- Abandoned buildings become dilapidated; they NEVER improve on their own. As the materials decay, they become more disordered until they fall apart.
- The tendency to disorder can even be applied to the state of your bedroom. The scene of an unmade bed with clothes and shoes strewn all over the floor is a classic example of entropy. It also shows the natural progression of energy. It takes very little energy for the disorder to progress but it takes a lot of energy on your part to create order by making the bed, hanging up the clothes, etc.
Once again we see that processes in nature are driven (or are spontaneous) toward least energy and greatest entropy.