Homogeneous and Heterogeneous

What is a mixture?
In chemistry, a mixture is a substance made up of 2 or more
substances that are not chemically combined but physically combined. This means
there are no chemical bonds between the different substances in a
homogeneous or heterogeneous mixture. You can think of a salad as an example of
a mixture— lettuce, tomatoes, cucumbers, and Parmesan cheese are the substances,
and each substance retains its chemical composition and identity.
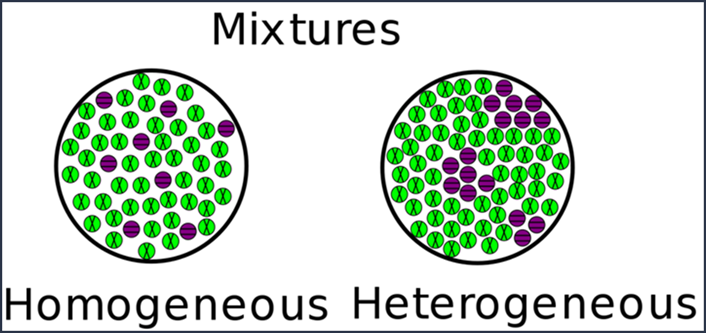
Definitions of homogeneous and heterogeneous mixtures
A homogeneous mixture (from the root “homo,” meaning same)
has a uniform composition. Furthermore, in a homogeneous mixture, all
substances exist in one state of matter. Liquids can be homogeneously mixed
with liquids, solids with solids, and so on.
On the other hand, a heterogeneous mixture (from the root
“hetero,” meaning different) has non-uniform composition, meaning that there
may be distinct regions with more or less of one component. Substances in a
heterogeneous mixture can exist in different states of matter at once – solid
with liquid or liquid with gas, for example.
Mixture Video
Defines a mixture as a combination of substances that can be
physically separated. Outlines the two main types of mixtures: homogeneous and
heterogeneous.
Examples of homogeneous mixtures
- Saltwater
If salt dissolves in water, it disperses evenly throughout the water. Note that seawater can be heterogeneous if pieces of particulate matter are present, as in nature. - Coffee, milk
These drinks contain many chemicals dissolved in water and spread evenly like saltwater. However, when milk curdles, it becomes a heterogeneous mixture. - Cement, glue
These are homogeneous mixtures of chemicals that set (harden) on drying or exposure to other special conditions. They may have other things added which could make them heterogeneous (see “Concrete” below) - Bronze, steel
These are alloys made by mixing copper and tin (for bronze) or iron and carbon (for steel). Because the resulting mixtures do not have different regions of each component, they are homogeneous. - Air
Air is a mixture of gases spread evenly throughout the atmosphere. Because gas molecules are distant from one another, they always mix evenly and do not form heterogeneous mixtures.
Examples of
heterogeneous mixtures
·
Sand
Sand usually consists of many
different types and sizes of particles, including different minerals and
pockets of air in between grains (or water if wet sand)
·
Oil and water
Most oils do not mix well with water,
so they have heterogeneous regions of mainly oil and mostly water
·
Salad
Salads contain many distinguishable
components: vegetables, cheese, chicken, dressing, or others
·
Granite
Granite, a common type of rock,
consists of grains of multiple minerals, like quartz, mica, and feldspar. The
grains are distinguishable, so this is a heterogeneous mixture.
·
Concrete
Concrete has pieces of gravel dispersed
in it which are distinguishable from the surrounding material (cement) that
holds them together. Sometimes these particles are as large as small stones.
·
Vegetable soup
Similar to a salad, a soup is not
homogeneous because there are many distinguishable parts. Even if you blend it
up, it will not be homogeneous on a molecular level.
·
Opened soda
An open soda is a heterogeneous
mixture because gases in the drink begin to come out of the solution when it
depressurizes. This results in the formation of gas bubbles distinguishable
from the surrounding drink.