Chemical Equations

Review: Elements and Compounds
The basic substances that chemists’ study is called chemical
elements.
Each element comprises tiny particles, or bits, called atoms.
Chemical reactions involve atoms or groups of atoms. When two or more atoms
combine, they form a molecule.
Each element has specific properties. When elements are
combined, they form a new substance with its properties. A substance formed in
this way is called a compound. There are a little more
than 100 elements. But there are millions of compounds.
Chemical Equations
Chemists write chemical equations to express the reactions
that form and break down molecules. These are shorthand versions of
ordinary word descriptions, and they make use of symbols and formulas for
elements and compounds.
For example:
Word description: Two molecules of hydrogen react with one molecule of oxygen
to form two water molecules.
Chemical equation: 2H2 + O2 → 2H2O
Word description: Two molecules of ammonia react to form one molecule of
nitrogen and three molecules of hydrogen.
Chemical equation: 2NH3 → N2 + 3H2
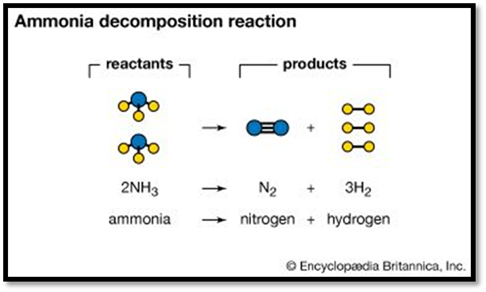
In chemical equations, the substances on the left side of the arrow—those
undergoing the chemical change—are called reactants. The substances on the right side—the result of the reaction—are
called products.
The arrow can be read as “give,” “form,” or “yield.”
Balancing Chemical
Equations
A chemical equation is a symbolic statement illustrating a chemical change.
In a chemical reaction, the number of atoms on the side of
the reactant should be equal to the number of atoms on the side
of the product.
First, to balance a chemical equation, balance the compound
with the most significant number of atoms.
Interpreting Chemical
Equations
A chemical reaction is when substances bond or break to produce new products.
Reactants will be on the left
side of the equation, and products will be on the right side.
The number before the chemical symbol indicates the number of
atoms.
The letters ”g” and “l” after the chemical symbols in an
equation indicate gas or liquid.
Video Illustration
The following video communicates how to balance and interpret
basic chemical equations. The program uses detailed animations and
illustrations to help aid in the understanding of basic chemistry.
Exploration
Practice balancing a chemical equation. In this Exploration, you
will be able to recognize the conservation of atoms and mass in a chemical
reaction, describe the difference between coefficients and subscripts in a
chemical equation, and translate from symbolic to molecular representations of
matter.