Unit 7:
Historical Models of the Atom

Unit Overview:
In
the last unit, you explored how chemists study matter through its interactions
by examining changes in matter, which are classified as either chemical or
physical. Recall that in unit 4, you learned that an element is a pure substance that is made up of a single type of
atom. Elements are often referred to as
the building blocks of matter because they are the smallest type of matter than
can exist on their own. The smallest particle of an element that can exist on
its own is an atom.
In
this unit, you will begin to dig more deeply into these particles, which make
up elements. You will explore the historical development of the model of the
atom.
What are the
origins of the concept of the atom?
The
concept of the atom began in philosophy.
A philosopher explains the
world around them through thinking and logic.
The concept of the atom was first considered in the 5th century BC. This concept is credited to the Greek
philosopher, Democritus, and the word atom is based on the Greek word “atomos,”
which literally translated means “not cut” and is interpreted to mean
indivisible. Democritus proposed that
the world was made up of the smallest piece of matter, that combines to form
other forms of matter. How did
Democritus develop this idea? Although
we cannot know exactly how he came up with this idea, you can imagine that
perhaps one day, he was lying under a tree, and he may have thought to himself:
“I can cut this tree down to gather wood for a fire. I can cut the tree into
smaller logs. I can cut the small
branches into kindling. I wonder if I
ever get to the point that I can no longer cut it into smaller pieces?” Then, he imagined the answer to his question
was, “Yes. Eventually, I will cut this into the smallest pieces of the tree
that can exist, and imagined the world was made up of the smallest particle,
some piece that he could ‘not cut,’ thereby theorizing the existence of the
atom.”
During
this time period, Democritus’ concept of the atom was shared by another Roman
philosopher, Leucippus. However, the philosopher, Aristotle, shared a competing
philosophy, in which he proposed the existence of 5 elements -- earth, water,
air, fire, and aether. The Aristoelian
perspective was more widely accepted.
What are the
reaction laws?
The
concept of the atom is revisited in the realm of science much later in
time. A scientist explains the world around them through thinking and logic
about experimental evidence. Two very
important laws that describe chemical reactions helped to ground the concept of
the atom in scientific theory.
1. The law of conservation of matter states that no matter can be created
or destroyed during any ordinary chemical reaction.
● Scientist: Antoine Lavoisier, 1789
● Summary of the experiment: Metals were burned in a
closed system. The total mass of the
system before and after the burning process was recorded.
● Experimental evidence: The beginning mass of the
system was the same as the ending mass of the system.
● Explanation: The total mass of the
system remains constant throughout a change.
Therefore, the same amount of matter that was present before the
reaction is present after the reaction.
This explanation became the law of conservation of matter after many
other scientists replicated the results.
2. The law of definite proportions states that the mass of one element
that combines with a fixed mass of another element to form a compound is always
the same. In other words, elements
combine in the same small whole-number ratios to form a compound.
● Scientist: Joseph Proust. 1804
● Summary of the experiment: Proust mathematically
compared the mass of elements that were used to synthesize carbon dioxide and
copper carbonate.
● Experimental evidence: The ratio of the masses of elements that
combined remained constant. That is,
when a smaller amount of 1 element was used, it also required a smaller amount
of the other element; when comparing the ratio of the masses that combined, the
ratio was always the same.
● Explanation: The constant ratio of
elements to form compounds indicates that elements always combine in the same
way to form a different substance. This explanation became the law of definite
proportions after many other scientists replicated the results.
3. The law of multiple proportions states that when two elements combine with each other to form
more than one compound, the weights of one element that combine with a fixed
weight of the other are in a ratio of small whole numbers.
● Scientist: John Dalton
● Summary: The existence of this third
reaction law was predicted by the 5 postulates of the atomic theory. [outlined
below] After proposing the postulates to explain the above 2 reaction laws,
Dalton predicted that if his postulates were correct, then the statement of the
law of multiple proportions should also be able to explain chemical reactions.
● Experimental evidence: After Dalton predicted its
existence, many scientists gathered experimental evidence that compared the
masses of elements that combine to form more than 1 compound. This evidence
showed that the mass ratios of elements between compounds were also a whole
number.
● Explanation: The whole number ratio of
elements between compounds indicates that elements must consist of the smallest
unit that combines differently with other atoms of different elements.
What is the
atomic theory?
Based
on the above 2 reaction laws, John Dalton proposed 5 postulates to explain why
these reaction laws described chemical reactions. These 5 postulates are now referred to as the
atomic theory.
Postulates of Dalton’s Atomic Theory:
1. All matter consists of tiny
particles called atoms.
2. Atoms of the same element
have identical properties; atoms of different elements have differing
properties.
3. Atoms cannot be subdivided,
created, or destroyed.
4. Atoms can combine in small
whole-number ratios to form compounds.
5. During chemical reactions,
atoms can be combined, separated, or rearranged.
This
theory both predicted the existence of the third reaction law and provided
scientific evidence of the existence of the atom.
Watch
the Atomic Theory video that is shown below to further consider the postulates
of Dalton’s Atomic Theory:
Practice
1: Take this
online quiz.
What are the
historical models of the atom?
The
experimental evidence was used to develop the model of the atom. Based on the reaction laws and Dalton’s
atomic theory, the first model of the atom is referred to as the spherical model of the atom.
|
What it looks like:
|
What it's based on: ● Law of conservation of matter ● Law of definite proportions ● Law of multiple proportions |
Why it explains the evidence: If matter cannot be created nor destroyed
and can only combine in small, whole-number ratios, then whole atoms must
react. |
However,
further experimental evidence was used to develop the model of the atom over time.
Many scientists and many experiments have contributed to our understanding of
the atom. Two of those historical
experiments are described below:
1.
The Cathode Ray Tube
Experiment
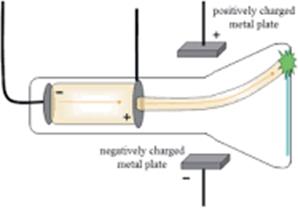
● Scientist:JJ Thomson, 1897
● Summary of the experiment: Cathode rays, light given
off by elements when an electrical current is passed through them, were placed
in a magnetic field.
● Experimental evidence: The cathode ray was
attracted to the positive end of the magnetic field.
● Explanation: The particles that make up
the cathode rays are conducting electricity and must be negatively charged.
● Discovery: The electron is a negatively charged particle that exists within the
atom.
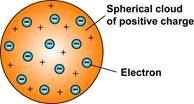
● Model of the atom: This evidence is described by Thomson’s plum pudding model of the atom.
|
What it looks like:
|
What it's based on: ● Thomson’s Cathode Ray Tube Experiment ● The discovery of the electron |
Why it explains the evidence: There is evidence of a negatively-charged
particle within the atom, but matter is also neutral, so the negative
particles are placed into a sea of a positive charge. |
In
order to help you visualize the experiment, please watch the following video:
2.
The Gold Foil Experiment
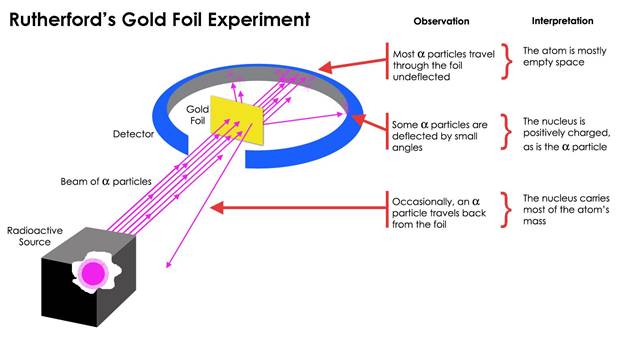
● Scientist: Ernest Rutherford, 1909
● Summary of the experiment: Alpha particles, highly
energetic, positively-charged particles were shot through gold foil, detecting
what happened to them.
● Experimental evidence: The majority of the alpha
particles went straight through the foil; a small percentage of them were
deflected at angles; a very few of them bounced back from the foil, never
making it through.
● Explanation: The majority of the alpha
particles that went straight through the foil did not encounter any other
charged particle within the foil. However, the small percentage that was
deflected at angles came close to a positive charge and the very few that did
not make it through the foil directly encountered a positive charge within the
foil.
● Discovery: The nucleus is a dense center of positive charge that exists within the
atom.
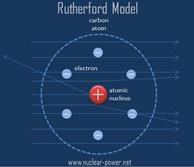
● Model of the atom: This evidence is described by Rutherford’s nuclear model of the atom.
|
What it looks like:
|
What it is based on: ● Rutherford’s Gold Foil Experiment ● The discovery of the nucleus |
Why it explains the evidence: Because so few alpha particles encountered a
positive charge within the foil, it cannot be a sea of positive charge, but
rather a dense center of a positive charge. |
In
order to help you visualize the experiment, please watch the following video:
Practice
2: Take this online quiz. (But you can ignore the last 3
questions - we’ll get to them in the next unit.)
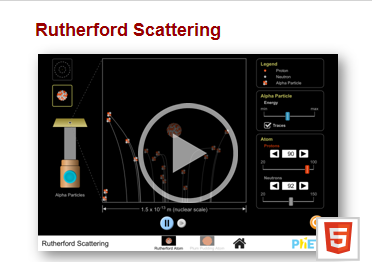
ChemLab: Rutherford Scattering Phet
Simulation
Overview:
The
results of Rutherford’s Gold Foil Experiment caused changes to the model of the
atom. In this lab, you will explore his
experiment, considering the variables that were tested.
Directions:
1. Download the Student
Exploration Sheet.
2. Practice using the Phet simulation.
3. Follow the instructions in the Exploration
Sheet to explore the relationships between the amount of solute and
solvent. Be sure to record your answers,
so that you can upload your completed lab sheet.