Unit 6: Changes
in Matter
Unit Overview:
In
the last 3 units, you considered how properties are used to describe matter and
how those properties help us to classify matter in two distinct systems:
1. Composition of Matter. You
classified matter according to its composition. You differentiated between pure
substances (elements & compounds) and mixtures (heterogeneous &
homogeneous).
2. Phases of Matter. You classified matter according to its
structure. You differentiated between
solids, liquids, and gases.
In
this unit, you will examine changes in matter.
If
you think back to the four ways in which chemists study matter, you will begin
considering the fourth way in this unit:
1. It's properties. Chemists describe matter – what it looks like
and how it behaves.
2. It's composition. Chemists
determine what components makeup samples of matter.
3. It's structure. Chemists
examine how the samples of matter are constructed.
4.
It's interactions. Chemists
study how samples of matter interact with each other.
You
will also notice that this unit is closely aligned with unit 3, in which you
explored properties of matter.
What is a
change in matter?
Chemists
describe changes in matter by observing and measuring the properties of the
substances, comparing them at the start and at the end. Changes
are defined as alterations of matter that result in a change in appearance
and/or identity of the substance.
What are the
two types of changes in matter?
These
changes in matter are categorized as physical changes or chemical changes.
A physical change is a change in matter
that does not alter the identity of the sample. Consider the folding of a piece
of paper - its size and shape changes by folding it, but it remains as
paper. Physical changes tend to be
reversible. In the example of the folded
paper, I can unfold the paper and notice that its identity has not changed.
A chemical
change is a change in matter that alters the identity of the sample.
Consider the burning of a piece of paper - I no longer have paper after I’ve
burned it. It is now ash with entirely
new properties. Chemical changes tend to
be nonreversible. In the example of the
burnt paper, I cannot turn the ashes back into paper.
Explore the BrainPOP Activity: Property Changes to deepen your thinking about the
differences between a physical and a chemical change. Be sure to watch the video, complete quiz,
and read all three Related Readings.
Practice 1: Differentiate between each of the changes of
matter by identifying whether it describes a physical or a chemical
change.
1.
Burning
gasoline in a car’s engine
2.
Ice
dissolving in a glass of lemonade
3.
A glass of
grape juice fermenting and changing into wine
4.
A log
burning in a fireplace
5.
A carton of milk going sour
6.
A cube of
sugar dissolving in water
7.
Boiling
water evaporating into the air
What are some
examples of physical changes?
A
physical change does not form a new substance! Examples of physical changes
include breaking matter into smaller pieces, changing the shape of a sample of
matter, phase changes, and dissolving a substance in water.
Why
is a phase change a physical change? Consider the freezing of water - an ice
cube is still made of water, but the water molecules are more rigidly
structured; an ice cube can melt again, and it returns to being water. The identity of the water stays the same, but
it looks different in the solid-state than in the liquid state, so it has
different properties. Phase changes are always physical changes.
Why
is dissolving a substance in the water a physical change? Consider adding salt
to water - the salt dissolves in the water, making it seem to disappear. However, we know that the salt is still
present because we can taste it in the water.
If we allow the water to evaporate, the salt is left behind. No new substance was formed.
Watch the Physical Changes of Matter video that is shown below to further consider examples of physical changes in matter:
What are some
examples of a chemical change?
A
chemical change forms a new substance!
Because new substance forms, the properties are changed.
A
chemical change is also called a chemical
reaction. Chemical reactions are
indicated by several common observations:
● Heat is given off.
● Light is given off.
● A precipitate forms. [A precipitate
is a new solid formed. It is observed as
cloudiness and/or solid particles falling to the bottom.]
● Bubbles form.
● An irreversible color change.
One
or more of these indicators occurring when 2 samples of matter interact
indicates that a chemical change has occurred.
Watch the Chemical Changes Warm-up video that is shown
below to further consider examples of physical changes in matter:
Practice
2: Complete the quiz.
What scientific law do all changes in
matter follow?
All
changes in matter obey the law of conservation of matter, which states that
matter cannot be created or destroyed. During a physical change, matter may be
moved closer together or further apart from each other, but no particles are
lost or created. During a chemical change, the atoms are rearranged to create
new substances.
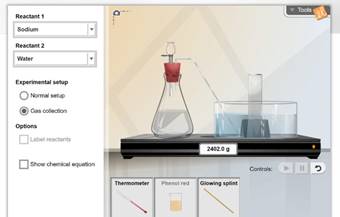
ChemLab
Chemical Changes
Overview:
In
this lab, you will be examining changes that occur in matter, focusing on
evidence of chemical changes.
Directions:
1.) Download the Chemical Changes
Student Exploration and Vocabulary
Sheets.
2.) Familiarize yourself with the words on the
vocabulary sheet.
3.) Log-in to your Explore Learning account.
4.) Click on “Chemical Changes” and launch the gizmo.
5.) Answer the Prior Knowledge Question.
6.) Practice using the Gizmo, using the Gizmo warm-up
instructions.
7.) After you are comfortable
using the Gizmo, begin the activity. Use the lab sheet as a guide to complete
the 3 activities:
Activity A: Observing
Chemical Changes of Matter
Activity B: Conservation of
Matter
Activity C: Types of
Reactions
