What is Chemistry?

Course Overview
Chemistry is the study of matter and the
changes they undergo. This is a vast
subject because EVERYTHING is matter!! Solids, Liquids, Gases, and everything
around you is matter. The only thing that isn't matter is ENERGY. Such as the
electricity your computer is using, the heat your body is giving off, the sound
coming from your mp3 player and the light and heat from the sun, these are all
examples of energy and are NOT matter.
What
is Chemistry?
Click the above picture to understand the
introduction of chemistry.
Types of Chemistry


Since chemistry is such a broad science, it is broken down
into different aspects of matter.
·
Organic
Chemistry - the study of substances that contain carbon
·
Biochemistry
- the study of the substances and chemical processes found in living things
·
Inorganic
Chemistry - the study of substances that do not contain carbon
·
Analytical
Chemistry - the study of the composition and structure of substances/matter
· Theoretical Chemistry – the study of using computers and mathematics to develop theories and mobels of molecular systems
· Physical Chemistry – the study of the principles of physics used in chemical interactions to study the behavior and interactions of matter at the molecular and atomic level
Let’s Practice -
Identify the correct
type of chemistry based on the description below.
1.) This is concerned with chemical processes and
substances that occur within living organisms.
2.) This is concerned with the
application of the techniques and theories of physics to the study of chemical
systems.
3.) This is concerned with the chemistry
of carbon compounds.
4.) This is concerned with the chemistry
that deals with inorganic compounds.
5.) This is concerned with using
instruments and methods to identify matter.
Qualitative and
Quantitative


Qualitative has to do with what the matter is
made of, think of the word "Quality."
·
No
numbers
Quantitative has to do with how much stuff is in
the matter...think of the word "Quantity."
·
Numbers
Let’s Practice – Identity as
either qualitative or quantitative.
6.) Determine whether the following
statement is about qualitative or quantitative data: The baby weighs 20 pounds.
7.) Determine whether the following
statement is about qualitative or quantitative data: My friend is thrilled.
8.) Determine whether the following
statement is about qualitative or quantitative data: The sky is greyish-blue.
9.) Determine whether the following statement is about
qualitative or quantitative data: Joe is 6 foot 2.
10.) Determine whether the following
statement is about qualitative or quantitative data: Diana has $100.
Levels of Study


Like all sciences, chemistry and its various branches fall
into two categories: Pure and Applied.
Pure science is science to increase mankind's
knowledge of the universe.
Applied science is taking that knowledge and making
our lives better.
·
Applied
science is better known as TECHNOLOGY, and we all love our technology.
Let’s Practice – Identify as
either Pure or Applied Science.
11.) Development of antibiotics
12.)
Study of the moon’s
phases
13.) Creating a shatter-resistant
glass
14.)
Observation of climate changes in the northeast part of Ohio
15.) Apply climate change data to
predict the impact of native Ohio wildlife
ChemLab


Enrollment Instruction to Virtual Lab
Throughout this course, you will explore chemistry in a
virtual lab setting. The virtual labs are
referred to as “Gizmos,” and the website is called Explore
Learning. So, to begin, you need to follow these instructions to enroll:
Follow
these simple steps to enroll to access these "Gizmos."
1. Contact your teacher to
enroll you in Gizmos.
2. Your teacher will send
you a "Student Login Card."
a. Look at the example
card below
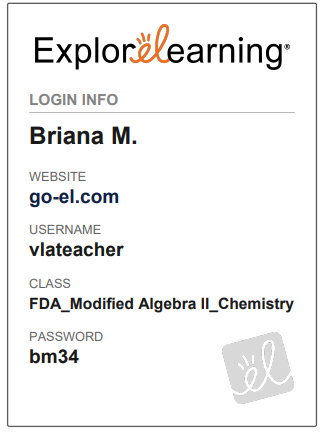
3.
Once you receive your "Student
Login Card," please click the link below and follow the instructions.
Login -
Student | ExploreLearning
|
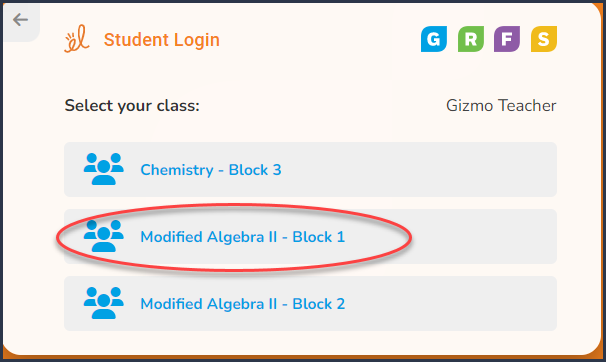
Step 1 |
Enter your teacher's
username: Located on Student Login
Card
|
|
Step 2 |
Select your class: Located on Student Login Card
|
|
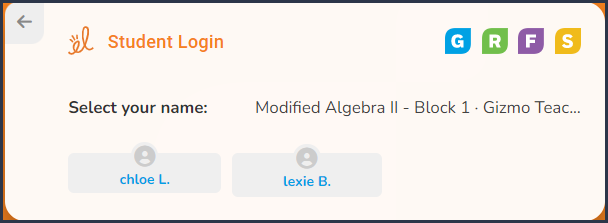
Step 3 |
Select your
name: It will be your first name with the first letter of your last name.
|
|
Step 4 |
Select
your product: Gizmos
|
|
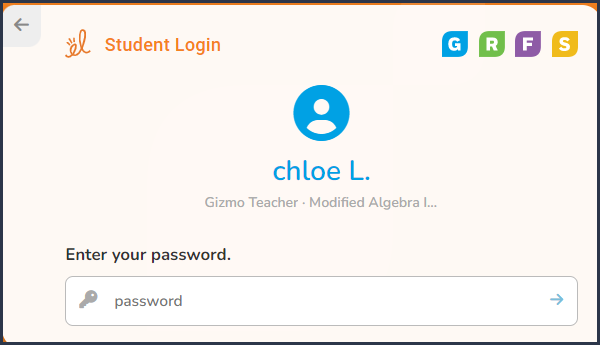
Step 5 |
Enter your
password: Password located on Student Login Card
|
|
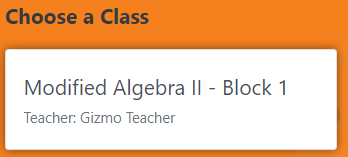
Step 6 |
Click on your course
to get to the Gizmos.
|
How does the virtual lab work?
When you work in the virtual lab, you will follow these
general directions:
1.
Download
the lab sheet and accompanying vocabulary sheet linked in your course.
2.
Familiarize
yourself with the words on the vocabulary sheet that will be used within the
lab activity.
3.
Click
on the title of the lab described in your lab sheet and launch the gizmo for
it.
4.
Always
begin with the Prior Knowledge Question and the Gizmo Warm-Up instructions.
a.
The
Prior Knowledge question is intended to help you connect your ideas/experiences
to the concept that you are examining.
b.
The
Gizmo Warm-Up instructions give you practice using the tools in the lab before
you begin.
5.
After
you are comfortable using the tools, begin the activity. Use the lab sheet as a
guide – it provides both step-by-step
directions and questions to answer as you conduct the lab. Decide how you want to work:
a.
You
can type your answers directly into the lab sheet that you download.
b.
You
can print the lab sheet and write your answers on it.
Now that you are enrolled, you will conduct your virtual lab!
Measuring Volume

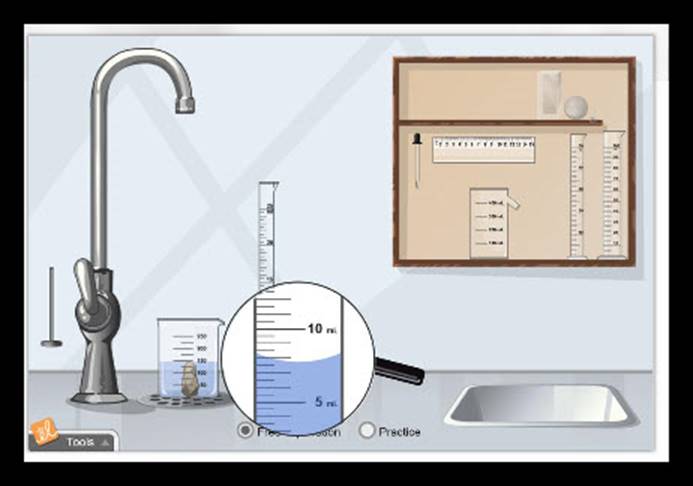
Overview:
In this lab,
you will be exploring the quantitative measurement of volume, using various
tools and measurements, depending on what is being measured.
Directions:
1) Download the Student Exploration:
Measuring Volume lab sheet and the Vocabulary: Measuring Volume.
2) Familiarize yourself with the words
on the vocabulary.
3) Log-in to your Explore Learning
account.
4) Click on “Measuring Volume” and launch the gizmo.
5) Answer the Prior Knowledge Question.
6) Practice using the Gizmo, using the
Gizmo warm-up instructions.
7) After you are comfortable using the
Gizmo, begin the activity. Use the lab sheet as a guide to complete the 3
activities:
a. Activity A: Volume of Liquids
b. Activity B: Regular Solids
c. Activity C: Water Displacement